Lead exposure-induced changes in hematology and biomarkers of hepatic injury: protective role of Trévo™ supplement
Article information
Abstract
Lead exposure has been linked to health challenges involving multiple organ failure. More than fifty percent of lead present in the human body is accumulated in the liver causing hepatic injury. A major mechanism of lead toxicity is oxidative stress. Trévo™ is a nutritional supplement with numerous bioactive natural products with detoxifying and antioxidant properties. This study was designed to investigate the hepatoprotective effects of Trévo™ dietary supplements against lead-hepatotoxicity in male Wistar rats. Thirty-five healthy animals were divided into five groups of seven each as follows: Group I=control; II=15 mg/kg of lead acetate (PbA); III= 2 mL/kg of Trévo™ + PbA; IV= 5 mL/kg of Trévo™ + PbA;V=5 mL/kg of Trévo™. Animals were orally treated with Trévo™ for two days before co-administration with PbA intraperitoneally for 12 consecutive days. Animals were sacrificed 24 h after the last administration and blood were collected via cardiac puncture and processed for hematological parameters and assessment of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB). The liver was excised and processed for markers of oxidative stress and histopathological examination. Intraperitoneal administration of 15 mg/kg of PbA caused a significant increase in serum concentration of AST, ALT, while the concentration of ALB was significantly decreased (P<0.001). PbA caused a significant reduction in packed cell volume, hemoglobin while the total white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were increased. Oxidative stress was significantly pronounced in the liver of rats exposed to PbA as observed in the high concentration of malonedialdehyde, decreased concentration of glutathione, the activity of catalase, superoxide dismutase, and glutathione-S-transferase. Pretreatment with Trévo™ was able to significantly prevent the anemic, oxidative damage, and hepatic injury initiated by PbA. Histological examination also corroborated the biochemical results. In conclusion, the study reveals that Trévo™ is effective in attenuating PbA-induced hepatotoxicity in male Wistar rats.
Introduction
The liver is one of the largest and most important organs of the human body; it is the main organ for the detoxification and metabolism of drugs. Other functions of the liver include nutritional homeostasis, cholesterol, and glucose metabolism, and synthesis of clotting factors [1,2]. These functions have exposed it to the toxic effect of drugs and chemicals as a result of ingestion or other means of exposure to the chemicals. These toxic agents can be deleterious to the role of the liver as a detoxifying organ [3]. This chemical works by attacking functional macromolecules such as lipid, protein, and nucleic acids, either through the generation of free radicals, depletion of antioxidant molecules, inflammation, and apoptosis [4–6]. Some distortion in liver tissue includes damage to the membrane of the hepatocyte and organelles leading to swelling, damage, and necrosis. Health challenges and death from liver disorders are on the rise globally [7]. Some of the common liver diseases include biliary disease, hepatitis B and C, and alcoholic liver disease. Lead is one of the most toxic heavy metals that humans are often exposed to, making it a metal of public health concern. Lead exposure has been reported to be toxic to most organs, such as the liver, kidney, heart, brain, testes, and hematopoietic tissues [8–13]. Exposure to lead can be through various routes-orally through ingesting food and water contaminated with lead, inhalation of polluted air, from dust, burning fuel, and fossil. Due to the colorless and odorless nature of lead, it persists for a longer time in the environment. It can only be detected at a very high concentration, a stage where it becomes harmful to the environment and living organisms including humans. Sources of lead poison include contaminated food and water, lead-containing paint and gasoline, industrial emission [2,14]. Mudipalli [15] reported that the liver is the main storage organ of lead accumulation. More than 33% of accumulated lead in the human body is found in the liver, followed by the kidney. The animal experiment involving lead hepatotoxicity has shown that lead exposure altered enzymes and molecules involved in the metabolism of xenobiotics, metabolism of cholesterol, and liver hyperplasia. The toxicity of lead has been reported to cause liver injury, osteoporosis, neurological disorders, and cardiovascular diseases [3]. The accepted mechanism of lead toxicity is oxidative stress [2–4,16,17]. This is often achieved by generating free radicals and depleting the antioxidant systems. Lead has been reported to replace the cations in enzymes and proteins resulting in loss of activities and functions respectively [18]. Some of the macromolecules oxidized by lead include lipids (measured as malonedialdehyde (MDA), reduced glutathione (GSH), and antioxidants such as superoxide dismutase (SOD) and catalase (SOD)). The role of natural products in combatting the toxic effects of heavy metals and other poisonous chemicals is on the rise. Since the major mechanism of lead toxicity is oxidative stress, natural products rich in antioxidants can be a good antidote against lead poison and can be used along with common lead chelators. Several compounds from natural products with confirmed antioxidant activities have been used as a hepatoprotective agent against lead position [2,3,7,16,17].
Trévo™ is a phytonutrient supplement that is manufactured in the USA under the trade name TRÉVO (TRÉVO LLC™) by United Int’l Lab LLC, TX 75244, USA. Trèvo formula contains potential antioxidants according to the manufacturer. It contains more than 174 ingredients extracted from various plant sources. The constituents involve many ingredients from phytonutrients of common garden fruits and vegetables, herbs, and coral calcium complex containing vitamins (A, C, and E), amino acids, trace minerals, essential fatty acids, digestive enzymes, and coenzymes, apart from primary essential antioxidants. Trévo™ is a mixture of various plant-rich extracts such as maqui berry, amalaki fruit, schizandra fruit, borojo fruit, goji fruit, noni, mangosteen, acai berry, gac fruit, camu camu, pomegranate, star fruit, and acerola cherry, antioxidant compounds such as beta-carotene, lycopene, ascorbic acid, α-tocopherol, and retinoic acid, essential fatty acids such as Omega-3 and Omega-6 from borage seed oil and flaxseed oil. All these substances have been reported to boost the immune system, cardiovascular functions, reproductive and nervous systems. In addition, these substances also have the potential to repair and regenerate new cells. According to the manufacturer, daily intake of Trévo™ provides the body with a high concentration of antioxidants that can prevent the generation of free radicals, thus countering or slowing down the effect of toxic chemicals and poison. Recent investigations show that Trévo™ was protective against acetaminophen, cyanide-induced liver damage [19,20], in addition to its neuroprotective and cardioprotective activities [21, 22]. This study investigated the effectiveness of Trévo™ to protect against lead-induced biochemical and histological changes in the liver of male Wistar rats.
Materials and Methods
Chemicals and reagents
Reduced glutathione, Trévo™ was a product of Trèvo™ LLC, Oklahoma City, USA. Other chemicals were of analytical grade.
Animal care and handling
Thirty-five male Wistar rats weighing 170±10g were purchased from the Central Animal House, University of Benin, Edo State, Nigeria were used for this experiment. The animals were housed in well-ventilated cages and provided water and food ad libitum.
Experimental design
Male Wistar rats were randomly divided into 5 groups of 7 rats each as follows:
Group 1: administered vehicle (distilled water)
Group 2: administered 15 mg/kg of lead acetate (PbA) intraperitoneally for 12 consecutive days [4].
Group 3: orally administered 2 mL/kg of Trévo™ for 2 days before co-administration with PbA for 12 consecutive days.
Group 4: orally administered 5 mL/kg of Trévo™ for 2 days before co-administration with PbA for 12 consecutive days.
Group 5: orally administered 5 mL/kg of Trévo™ for 14 consecutive days.
Processing of the liver
24 h after last administration, animals were sacrificed via cervical dislocation, and the liver excised, rinsed, and homogenized in a phosphate buffer saline (0.1M, pH 7.4) to obtain a 10% w/v homogenate. The homogenate was centrifuged at 15000 rpm for 10 min with the temperature set at 4 °C to obtain a clear supernatant that was used for biochemical assays.
Biomarkers of liver function
Serum activity of alanine aminotransferase, aspartate aminotransferase, and concentration of albumin was determined in the serum following the instruction from the kit manual.
Biochemical assay
Estimation of oxidants in the hepatic tissues
Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances according to the method of Varshney and Kale [23]. The malondialdehyde level was calculated using a molar extinction coefficient of 1.56 × 105 M −1 cm −1.
Estimation of antioxidants in the hepatic tissues
GSH content was estimated according to the method of Jollow et al. [24]. The reaction is based on the fact that the thiol group of GSH reacts with 5, 5′-dithiobis-(2-nitrobenzoic acid) to form thionitrobenzoic acid, which was monitored at 412 nm. Catalase (CAT) activity was determined as described by [25]. Superoxide dismutase (SOD) activity was evaluated by the method of Misra and Fridovich [26] by monitoring the absorbance of a mixture containing the brain supernatant, carbonate buffer (0.05 M, pH 10.2), and adrenalin (0.3 mM) for 150 s at 480 nm.
GST assay
Glutathione transferase (GST) activity was determined by measuring the conjugation of GSH with 1-chloro-2, 4-dinitrobenzene at 340 nm as described by Habig et al. [27].
Histopathological evaluation
Liver tissues were taken from the eviscerated rats and fixed in 10% formalin for 24 h, and then processed to obtain paraffin blocks. Sections of 4–6 μm thickness were cut using a microtome and stained with hematoxylin and eosin (H and E) stain by using the method of Stevens and Wilson [28].
Statistical analysis
All grouped data were statistically performed with Prism (GraphPad Prism, 6.01) software. Differences among groups were evaluated by one-way analysis of variance followed by Duncan’s multiple range tests. All values were expressed as the mean±standard deviation of seven animals per group.
Results
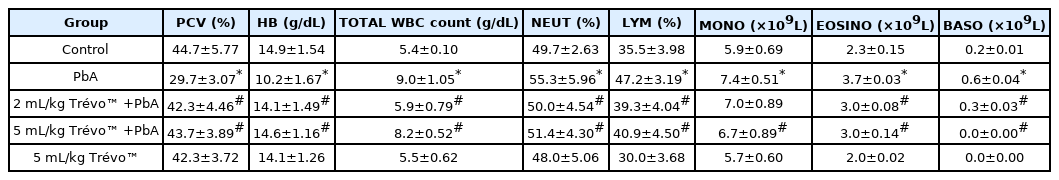
Effect of lead acetate (PbA) and pretreatment with Trévo™ (2 and 5 mL/kg) on hematological parameters in male Wistar rats
Table 1 shows that PbA caused a significant decrease in PCV and a mild decrease in Hb as compared to the control (P<0.05), while the level of WBC, Neu, lymph, mono, eosin, and baso significantly increased when compared with the control (P<0.05). Pretreatment with Trévo™ (2-and 5 mL/kg) was able to prevent the anemic and inflammation induced by PbA as observed in the significant increase in PCV and Hb level as compared to the untreated group (P<0.05). In addition, the level of other blood parameters (Neu, lymph, mono, eosin, and baso) was significantly decreased as compared to the untreated group (P<0.05). Administration of the rats with 5 mL/kg of Trévo™ had no significant effect on hematology parameters when compared to the control (P<0.05)
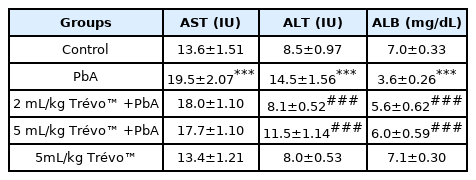
Effect of lead acetate (PbA) and pretreatment with Trévo™ (2 and 5 mL/kg) in serum markers of hepatotoxicity
Table 2 shows that exposure of rats to lead acetate caused a significant increase in AST, ALT, and ALB levels as compared to the control group (P<0.001). Pretreatment of the rat with 2- and 5 mL/kg of Trévo™ reduced the hepatotoxic effect of PbA as observed in the significant decrease in the serum level of AST, ALT, and ALB when compared to PbA (P<0.05). Treatment with 5 mL/kg of Trévo™ had no significant effect on serum markers of hepatotoxicity when compared to the control (P>0.05).
Effect of PbA and Trévo™ on markers of oxidative stress
Figure 1 and 2 shows the effect of PbA and pretreatment with Trévo™ on the concentration of malonedialdehyde (MDA) and reduced glutathione (GSH) respectively.
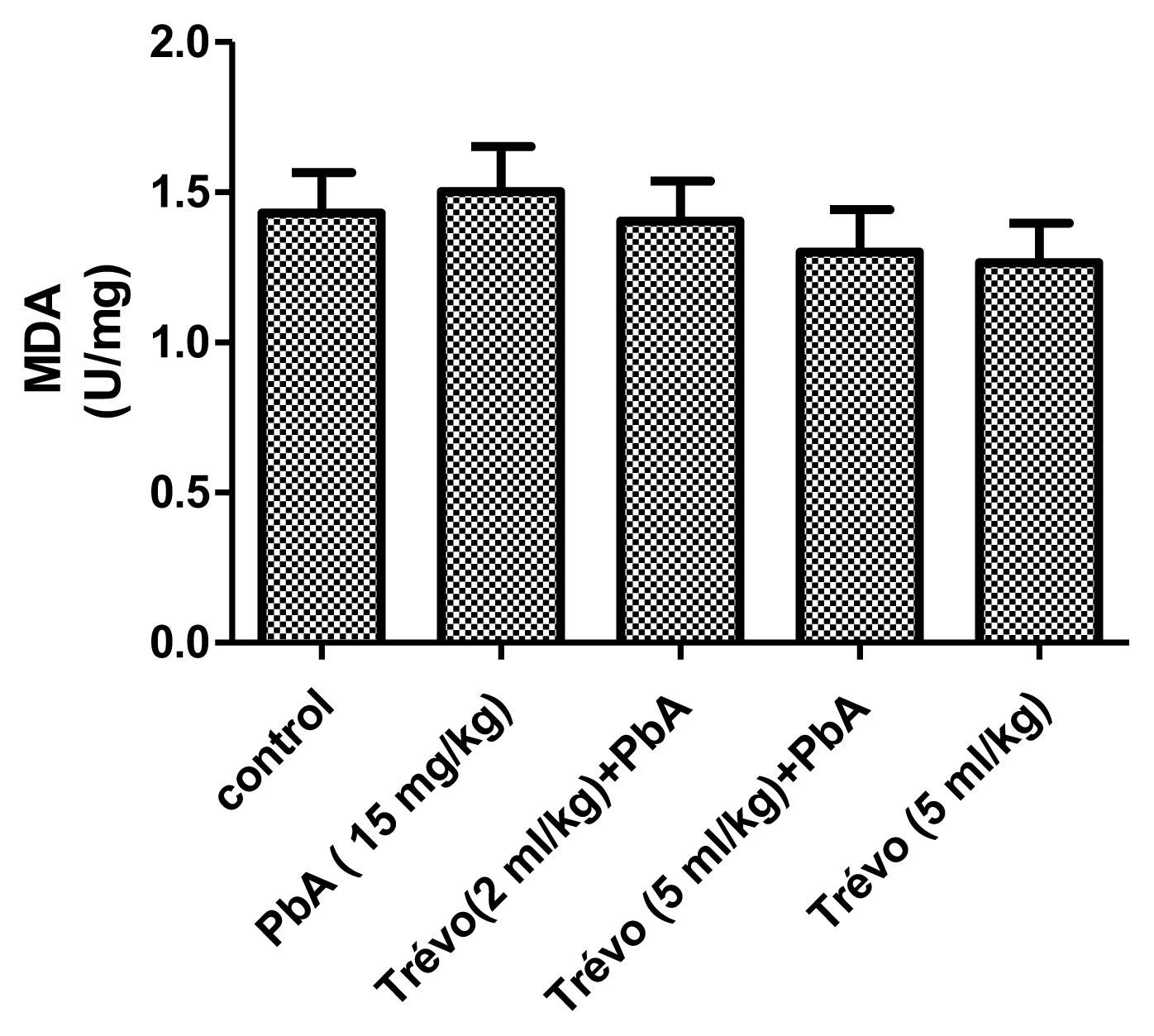
The concentration of lipid peroxides product - malondialdehyde (MDA), in the liver tissue of male rats after 2 day-pretreatment with Trévo™ and 12-day co-administration with lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals.
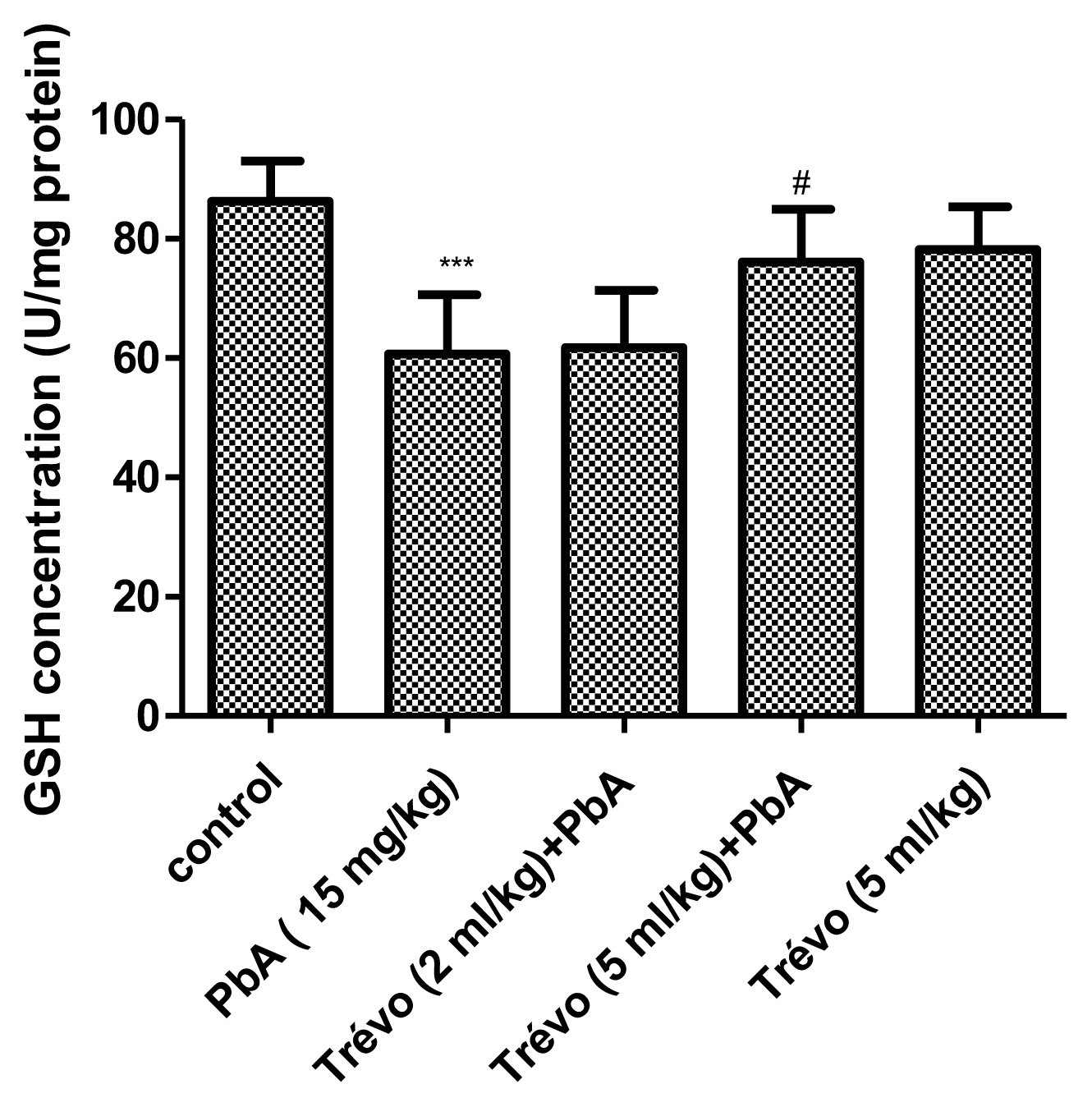
The concentration of non-enzymatic antioxidant-reduced glutathione (GSH) in the liver tissue of male rats after 2 day-pretreatment with Trévo™ and 12-day co-administration with lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; #P<0.05= PbA vs. Trévo™ (5 mL/kg) + PbA
PbA caused an insignificant increase (P<0.05) in the level of MDA and a significant decrease (P<0.05) in the level of GSH when compared to the control. Pretreatment with Trévo™ (2- and 5 mL/kg) was able to reduce the level of MDA, though not significantly different when compared to the untreated group (P>0.05). For GSH, pretreatment of the rats with 2 mL/kg of Trévo™ had no significant effect on GSH concentration, when compared to the untreated group (P>0.05), while pretreatment with 5 mL/kg of Trévo™ caused a significant increase in the concentration of GSH when compared to the untreated group (P<0.05). Administration of 5 mL/kg of Trévo™ only to the rats had no significant effect on GSH concentration when compared to the control group (P>0.05).
Figures 3 and 4 show the effect of PbA and pretreatment with Trévo™ on catalase (CAT) and superoxide dismutase (SOD) activities respectively. PbA caused a significant decrease in CAT and SOD activities as compared to the control (P<0.001 and 0.05 respectively). Pretreatment with 2- and 5 mL/kg of Trévo™ was able to prevent the inhibitory effect of PbA on CAT and SOD as observed in the significant increase activity of CAT and SOD as compared to the untreated group (P<0.05 and 0.001 respectively). The result also showed that a high dose of Trévo™ was more effective in increasing the activity of CAT and SOD than a low dose. Administration of rats with 5 mL/kg of Trévo™ had no significant effect on the activities of CAT and SOD as compared to the control (P>0.05).
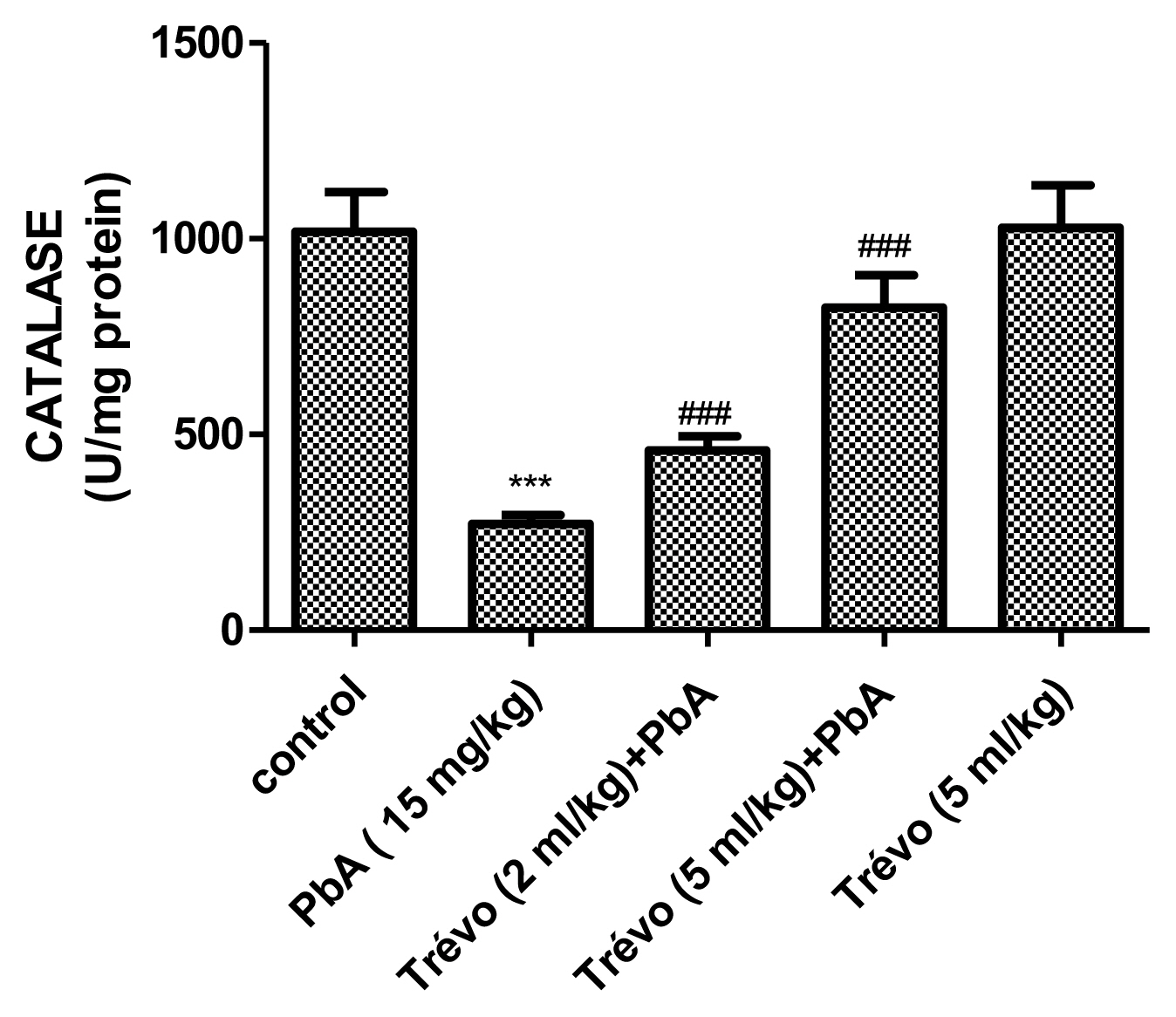
The catalase activity in the liver tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; ###P<0.001= PbA vs. treatment groups
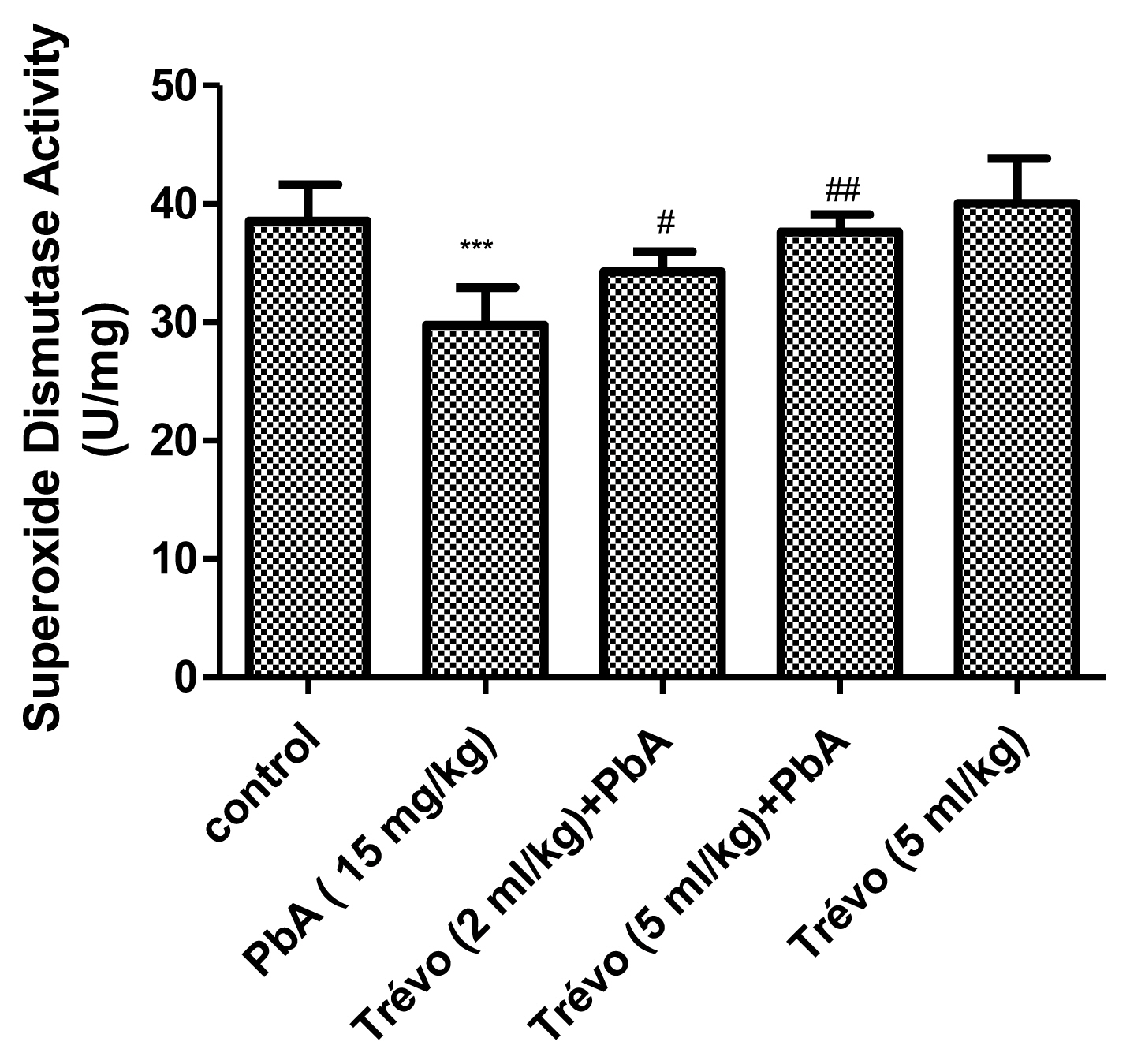
The superoxide dismutase (SOD) activity in the kidney tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/Kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; #P<0.05= PbA vs. Trévo™ (2 mL/kg) + PbA; ##P<0.01= PbA vs. Trévo™ (5 mL/kg) + PbA
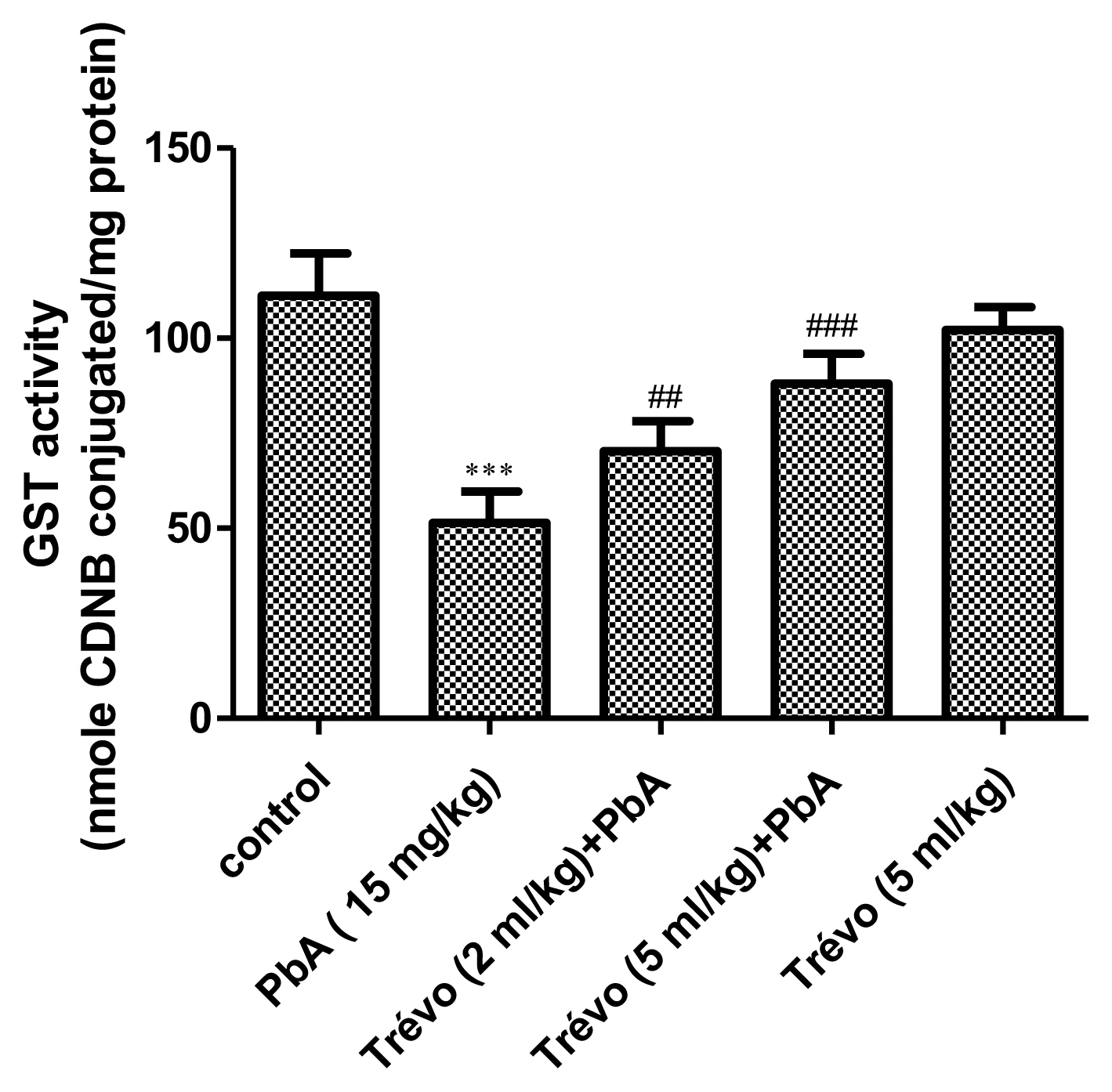
The glutathione-S-transferase (GST) activity in the liver tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; ##P<0.01= PbA vs. Trévo™ (2 mL/kg) + PbA; ###P<0.001= PbA vs. Trévo™ (5 mL/kg) + PbA
Effect of pretreatment with Trévo™ and lead acetate (PbA) on glutathione-S-transferase (GST) activity
PbA caused a significant decrease in the activity of GST as compared to control (P<0.001). However, pretreatment with 2-and 5 mL/kg of Trévo™ significantly increase the activity of GST as compared to the PbA group (P<0.05). An interesting observation is a significant decrease in the GST activity in animals administered only Trévo™ when compared to the control (P<0.05).
Histology
Liver from PbA administered rats was examined for histopathological changes. As shown in Figure 6 rats administered PbA only shows diffuse hepatic vacuolation with periportal hepatic necrosis and cellular infiltration, a situation that was ameliorated in the group pretreated with Trévo™ 2-and 5 mL/kg) before the co-administration with PbA with very mild portal necrosis.
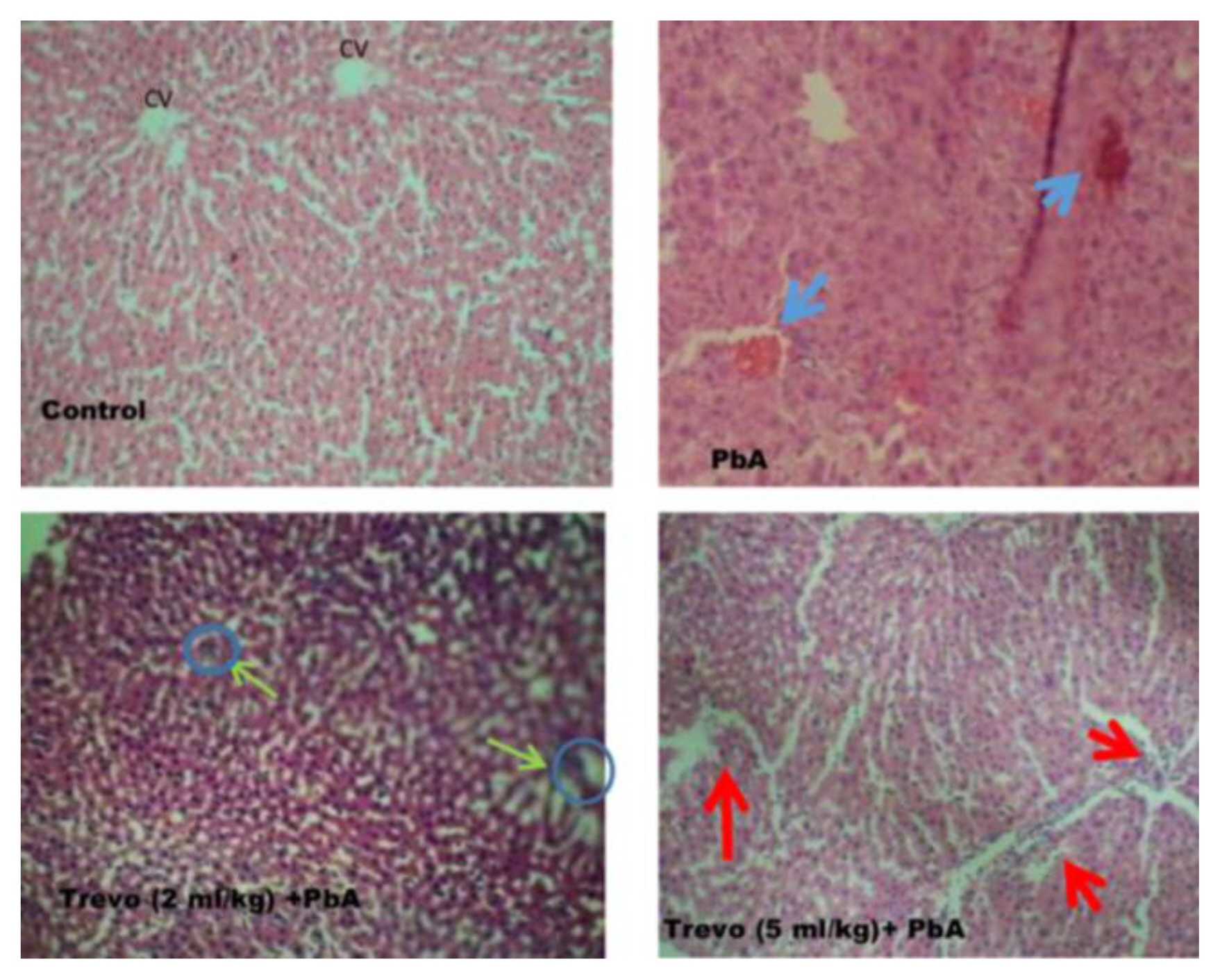
Photomicrograph of rat liver sections stained with hematoxylin and eosin (X100) showing alterations in the liver after treatment with trévo and exposure to PbA. The blue arrow indicates congestion. The green arrow indicates restoration and normal architecture of hepatic cords. The red arrows indicate normal hepatic artery and portal vein.
Discussion
The hepatotoxicity of PbA has been linked to its ability to form a complex with some proteins abundant in the liver. In addition, the liver is one of the major organs involved in Pb excretion. We hypothesized that Trévo™ a supplement drink that contains numerous phytochemicals with metal chelating properties can prevent lead hepatotoxicity. Our results show that Trévo™ at the two doses based on the method of [19] was able to prevent PbA-induced hepatotoxicity. The anemic effect of PbA as observed in the low percentage of PCV and hemoglobin was reversed by treatment with Trévo™, which was able to increase the percentage of PCV and Hb volume. Treatment with Trévo™ also reduced the total WBC and other blood parameters such as neutrophil, lymphocyte, monocyte, eosinophil, and basophil. A decrease in Hb content by PbA has been linked to the ability of PbA to inhibit erythropoiesis or its binding to RBC, which often results in low Hb content and short life for RBC respectively. Another anemic effect of PbA can also be due to its displacement of Fe from Hb or inhibiting the activity of aminolevulinic acid dehydratase (ALAD), all culminating in the reduction of Hb and lifespan of RBCs. The anemic effect of toxic heavy metals has been reported by various investigators and all supported our findings [28–32]. PbA administration for 10 days caused a significant increase in serum levels of AST ad ALT. The increased level of these enzymes might be due to leakage from the tissue as a result of damage caused by PbA. This observation is similar to the report of [2,3]. They reported that elevated serum levels of ALT and AST can be linked to damage of the membrane due to oxidative stress induced by PbA. This increased the permeability of the enzymes from the hepatocytes into blood circulation. In addition, PbA exposure also caused a significant decrease in serum albumin. Albumin plays an important role in blood detoxification. The depletion of albumin can further enhance the toxicity of PbA. One of the ways by which PbA caused albumin reduction is through forming a complex with the protein, thereby making it lose its biological function [33,34]. Pretreatment with Trévo™ before concurrent administration of PbA caused a significant reduction in the serum level of AST ad ALT and increase concentration of albumin. The hepatoprotective effect of Trévo™ against cyanide ad acetaminophen-induced liver injury has been reported [21,22]. Trévo ameliorated the hepatotoxicity of PbA by preventing damage to the hepatic membrane and leakage of the enzyme into the blood. In addition, it is possible that Trévo™ chelate PbA, thus preventing it from binding to a functional molecule such as albumin. Our result also supported the oxidative stress-induced mechanism of PbA as reported by other researchers [2–4,16,17]. PbA exposure caused a mild increase in MDA level as well as a significant decrease in GSH concentration, CAT, SOD, and GST activities in liver tissue. The mechanism of PbA-induced oxidative stress involves, might involve increased production of ROS as a result of damage to enzymes involved in mitochondria respiration. The ROS generated react with biomolecules increasing peroxide products such as lipid peroxide ad protein carbonyl, further stressing the antioxidant system, as observed in the low level of CAT, SOD, and GST. The histopathological examination of the liver tissue in rats administered PbA corroborates the findings of the biochemical assays. Morphological changes observed include congestion of the central venules, hepatic necrosis, and abscess in the liver parenchyma decreased portal triads and necrotized hepatocytes. Pretreatment with Trévo™ prevents the morphological changes observed. Some of the phytochemicals present in Trévo™ have been reported to improve liver functions and the purification of the organ. Pomegranate, Amalaki, and Phyllanthus emblica [36,37]. These are compounds with established antioxidant and hepatoprotective properties. Therefore, the ability of Trévo™ to prevent enzyme leakages and the oxidative effect of PbA might not be unconnected with the phytochemicals present in the product.
Conclusions
In conclusion, our results show that Trévo™ was hepatoprotective against lead toxicity and the activity might be linked to the presence of numerous antioxidants phytochemicals present in Trévo™.
Acknowledgement
The authors will appreciate some of the students who assisted in conducting the experiment.
Notes
The authors declare no competing interest.
CRediT author statement
OBI: Conceptualization, Methodology, Investigation, Data Curation, Writing-Original draft preparation, EFA: Writing-Review & Editing, Formal analysis; TTO: Conceptualization, Data curation; BC: Resources