Ilesanmi, Adeogun, Odewale, and Chikere: Lead exposure-induced changes in hematology and biomarkers of hepatic injury: protective role of Trévo™ supplement
Abstract
Lead exposure has been linked to health challenges involving multiple organ failure. More than fifty percent of lead present in the human body is accumulated in the liver causing hepatic injury. A major mechanism of lead toxicity is oxidative stress. Trévo™ is a nutritional supplement with numerous bioactive natural products with detoxifying and antioxidant properties. This study was designed to investigate the hepatoprotective effects of Trévo™ dietary supplements against lead-hepatotoxicity in male Wistar rats. Thirty-five healthy animals were divided into five groups of seven each as follows: Group I=control; II=15 mg/kg of lead acetate (PbA); III= 2 mL/kg of Trévo™ + PbA; IV= 5 mL/kg of Trévo™ + PbA;V=5 mL/kg of Trévo™. Animals were orally treated with Trévo™ for two days before co-administration with PbA intraperitoneally for 12 consecutive days. Animals were sacrificed 24 h after the last administration and blood were collected via cardiac puncture and processed for hematological parameters and assessment of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB). The liver was excised and processed for markers of oxidative stress and histopathological examination. Intraperitoneal administration of 15 mg/kg of PbA caused a significant increase in serum concentration of AST, ALT, while the concentration of ALB was significantly decreased (P<0.001). PbA caused a significant reduction in packed cell volume, hemoglobin while the total white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were increased. Oxidative stress was significantly pronounced in the liver of rats exposed to PbA as observed in the high concentration of malonedialdehyde, decreased concentration of glutathione, the activity of catalase, superoxide dismutase, and glutathione-S-transferase. Pretreatment with Trévo™ was able to significantly prevent the anemic, oxidative damage, and hepatic injury initiated by PbA. Histological examination also corroborated the biochemical results. In conclusion, the study reveals that Trévo™ is effective in attenuating PbA-induced hepatotoxicity in male Wistar rats.
Keywords: Trévo™, lead, hepatotoxicity, oxidative stress, hematology
Introduction
The liver is one of the largest and most important organs of the human body; it is the main organ for the detoxification and metabolism of drugs. Other functions of the liver include nutritional homeostasis, cholesterol, and glucose metabolism, and synthesis of clotting factors [ 1, 2]. These functions have exposed it to the toxic effect of drugs and chemicals as a result of ingestion or other means of exposure to the chemicals. These toxic agents can be deleterious to the role of the liver as a detoxifying organ [ 3]. This chemical works by attacking functional macromolecules such as lipid, protein, and nucleic acids, either through the generation of free radicals, depletion of antioxidant molecules, inflammation, and apoptosis [ 4– 6]. Some distortion in liver tissue includes damage to the membrane of the hepatocyte and organelles leading to swelling, damage, and necrosis. Health challenges and death from liver disorders are on the rise globally [ 7]. Some of the common liver diseases include biliary disease, hepatitis B and C, and alcoholic liver disease. Lead is one of the most toxic heavy metals that humans are often exposed to, making it a metal of public health concern. Lead exposure has been reported to be toxic to most organs, such as the liver, kidney, heart, brain, testes, and hematopoietic tissues [ 8– 13]. Exposure to lead can be through various routes-orally through ingesting food and water contaminated with lead, inhalation of polluted air, from dust, burning fuel, and fossil. Due to the colorless and odorless nature of lead, it persists for a longer time in the environment. It can only be detected at a very high concentration, a stage where it becomes harmful to the environment and living organisms including humans. Sources of lead poison include contaminated food and water, lead-containing paint and gasoline, industrial emission [ 2, 14]. Mudipalli [ 15] reported that the liver is the main storage organ of lead accumulation. More than 33% of accumulated lead in the human body is found in the liver, followed by the kidney. The animal experiment involving lead hepatotoxicity has shown that lead exposure altered enzymes and molecules involved in the metabolism of xenobiotics, metabolism of cholesterol, and liver hyperplasia. The toxicity of lead has been reported to cause liver injury, osteoporosis, neurological disorders, and cardiovascular diseases [ 3]. The accepted mechanism of lead toxicity is oxidative stress [ 2– 4, 16, 17]. This is often achieved by generating free radicals and depleting the antioxidant systems. Lead has been reported to replace the cations in enzymes and proteins resulting in loss of activities and functions respectively [ 18]. Some of the macromolecules oxidized by lead include lipids (measured as malonedialdehyde (MDA), reduced glutathione (GSH), and antioxidants such as superoxide dismutase (SOD) and catalase (SOD)). The role of natural products in combatting the toxic effects of heavy metals and other poisonous chemicals is on the rise. Since the major mechanism of lead toxicity is oxidative stress, natural products rich in antioxidants can be a good antidote against lead poison and can be used along with common lead chelators. Several compounds from natural products with confirmed antioxidant activities have been used as a hepatoprotective agent against lead position [ 2, 3, 7, 16, 17]. Trévo™ is a phytonutrient supplement that is manufactured in the USA under the trade name TRÉVO (TRÉVO LLC™) by United Int’l Lab LLC, TX 75244, USA. Trèvo formula contains potential antioxidants according to the manufacturer. It contains more than 174 ingredients extracted from various plant sources. The constituents involve many ingredients from phytonutrients of common garden fruits and vegetables, herbs, and coral calcium complex containing vitamins (A, C, and E), amino acids, trace minerals, essential fatty acids, digestive enzymes, and coenzymes, apart from primary essential antioxidants. Trévo™ is a mixture of various plant-rich extracts such as maqui berry, amalaki fruit, schizandra fruit, borojo fruit, goji fruit, noni, mangosteen, acai berry, gac fruit, camu camu, pomegranate, star fruit, and acerola cherry, antioxidant compounds such as beta-carotene, lycopene, ascorbic acid, α-tocopherol, and retinoic acid, essential fatty acids such as Omega-3 and Omega-6 from borage seed oil and flaxseed oil. All these substances have been reported to boost the immune system, cardiovascular functions, reproductive and nervous systems. In addition, these substances also have the potential to repair and regenerate new cells. According to the manufacturer, daily intake of Trévo™ provides the body with a high concentration of antioxidants that can prevent the generation of free radicals, thus countering or slowing down the effect of toxic chemicals and poison. Recent investigations show that Trévo™ was protective against acetaminophen, cyanide-induced liver damage [ 19, 20], in addition to its neuroprotective and cardioprotective activities [ 21, 22]. This study investigated the effectiveness of Trévo™ to protect against lead-induced biochemical and histological changes in the liver of male Wistar rats.
Materials and Methods
Chemicals and reagents
Reduced glutathione, Trévo™ was a product of Trèvo™ LLC, Oklahoma City, USA. Other chemicals were of analytical grade.
Animal care and handling
Thirty-five male Wistar rats weighing 170±10g were purchased from the Central Animal House, University of Benin, Edo State, Nigeria were used for this experiment. The animals were housed in well-ventilated cages and provided water and food ad libitum.
Experimental design
Male Wistar rats were randomly divided into 5 groups of 7 rats each as follows:
Group 1: administered vehicle (distilled water) Group 2: administered 15 mg/kg of lead acetate (PbA) intraperitoneally for 12 consecutive days [4]. Group 3: orally administered 2 mL/kg of Trévo™ for 2 days before co-administration with PbA for 12 consecutive days. Group 4: orally administered 5 mL/kg of Trévo™ for 2 days before co-administration with PbA for 12 consecutive days. Group 5: orally administered 5 mL/kg of Trévo™ for 14 consecutive days.
Processing of the liver
24 h after last administration, animals were sacrificed via cervical dislocation, and the liver excised, rinsed, and homogenized in a phosphate buffer saline (0.1M, pH 7.4) to obtain a 10% w/v homogenate. The homogenate was centrifuged at 15000 rpm for 10 min with the temperature set at 4 °C to obtain a clear supernatant that was used for biochemical assays.
Biomarkers of liver function
Serum activity of alanine aminotransferase, aspartate aminotransferase, and concentration of albumin was determined in the serum following the instruction from the kit manual.
Biochemical assay
Estimation of oxidants in the hepatic tissues
Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances according to the method of Varshney and Kale [ 23]. The malondialdehyde level was calculated using a molar extinction coefficient of 1.56 × 105 M −1 cm −1.
Estimation of antioxidants in the hepatic tissues
GSH content was estimated according to the method of Jollow et al. [ 24]. The reaction is based on the fact that the thiol group of GSH reacts with 5, 5′-dithiobis-(2-nitrobenzoic acid) to form thionitrobenzoic acid, which was monitored at 412 nm. Catalase (CAT) activity was determined as described by [ 25]. Superoxide dismutase (SOD) activity was evaluated by the method of Misra and Fridovich [ 26] by monitoring the absorbance of a mixture containing the brain supernatant, carbonate buffer (0.05 M, pH 10.2), and adrenalin (0.3 mM) for 150 s at 480 nm.
GST assay
Glutathione transferase (GST) activity was determined by measuring the conjugation of GSH with 1-chloro-2, 4-dinitrobenzene at 340 nm as described by Habig et al. [ 27].
Histopathological evaluation
Liver tissues were taken from the eviscerated rats and fixed in 10% formalin for 24 h, and then processed to obtain paraffin blocks. Sections of 4–6 μm thickness were cut using a microtome and stained with hematoxylin and eosin (H and E) stain by using the method of Stevens and Wilson [ 28].
Statistical analysis
All grouped data were statistically performed with Prism (GraphPad Prism, 6.01) software. Differences among groups were evaluated by one-way analysis of variance followed by Duncan’s multiple range tests. All values were expressed as the mean±standard deviation of seven animals per group.
Results
Effect of lead acetate (PbA) and pretreatment with Trévo™ (2 and 5 mL/kg) on hematological parameters in male Wistar rats
Table 1 shows that PbA caused a significant decrease in PCV and a mild decrease in Hb as compared to the control (P<0.05), while the level of WBC, Neu, lymph, mono, eosin, and baso significantly increased when compared with the control (P<0.05). Pretreatment with Trévo™ (2-and 5 mL/kg) was able to prevent the anemic and inflammation induced by PbA as observed in the significant increase in PCV and Hb level as compared to the untreated group (P<0.05). In addition, the level of other blood parameters (Neu, lymph, mono, eosin, and baso) was significantly decreased as compared to the untreated group (P<0.05). Administration of the rats with 5 mL/kg of Trévo™ had no significant effect on hematology parameters when compared to the control (P<0.05)
Effect of lead acetate (PbA) and pretreatment with Trévo™ (2 and 5 mL/kg) in serum markers of hepatotoxicity
Table 2 shows that exposure of rats to lead acetate caused a significant increase in AST, ALT, and ALB levels as compared to the control group (P<0.001). Pretreatment of the rat with 2- and 5 mL/kg of Trévo™ reduced the hepatotoxic effect of PbA as observed in the significant decrease in the serum level of AST, ALT, and ALB when compared to PbA (P<0.05). Treatment with 5 mL/kg of Trévo™ had no significant effect on serum markers of hepatotoxicity when compared to the control (P>0.05).
Effect of PbA and Trévo™ on markers of oxidative stress
Figure 1 and 2 shows the effect of PbA and pretreatment with Trévo™ on the concentration of malonedialdehyde (MDA) and reduced glutathione (GSH) respectively.
PbA caused an insignificant increase (P<0.05) in the level of MDA and a significant decrease (P<0.05) in the level of GSH when compared to the control. Pretreatment with Trévo™ (2- and 5 mL/kg) was able to reduce the level of MDA, though not significantly different when compared to the untreated group (P>0.05). For GSH, pretreatment of the rats with 2 mL/kg of Trévo™ had no significant effect on GSH concentration, when compared to the untreated group (P>0.05), while pretreatment with 5 mL/kg of Trévo™ caused a significant increase in the concentration of GSH when compared to the untreated group (P<0.05). Administration of 5 mL/kg of Trévo™ only to the rats had no significant effect on GSH concentration when compared to the control group (P>0.05).
Figures 3 and 4 show the effect of PbA and pretreatment with Trévo™ on catalase (CAT) and superoxide dismutase (SOD) activities respectively. PbA caused a significant decrease in CAT and SOD activities as compared to the control (P<0.001 and 0.05 respectively). Pretreatment with 2- and 5 mL/kg of Trévo™ was able to prevent the inhibitory effect of PbA on CAT and SOD as observed in the significant increase activity of CAT and SOD as compared to the untreated group (P<0.05 and 0.001 respectively). The result also showed that a high dose of Trévo™ was more effective in increasing the activity of CAT and SOD than a low dose. Administration of rats with 5 mL/kg of Trévo™ had no significant effect on the activities of CAT and SOD as compared to the control (P>0.05).
Effect of pretreatment with Trévo™ and lead acetate (PbA) on glutathione-S-transferase (GST) activity
PbA caused a significant decrease in the activity of GST as compared to control (P<0.001). However, pretreatment with 2-and 5 mL/kg of Trévo™ significantly increase the activity of GST as compared to the PbA group (P<0.05). An interesting observation is a significant decrease in the GST activity in animals administered only Trévo™ when compared to the control (P<0.05).
Histology
Liver from PbA administered rats was examined for histopathological changes. As shown in Figure 6 rats administered PbA only shows diffuse hepatic vacuolation with periportal hepatic necrosis and cellular infiltration, a situation that was ameliorated in the group pretreated with Trévo™ 2-and 5 mL/kg) before the co-administration with PbA with very mild portal necrosis.
Discussion
The hepatotoxicity of PbA has been linked to its ability to form a complex with some proteins abundant in the liver. In addition, the liver is one of the major organs involved in Pb excretion. We hypothesized that Trévo™ a supplement drink that contains numerous phytochemicals with metal chelating properties can prevent lead hepatotoxicity. Our results show that Trévo™ at the two doses based on the method of [ 19] was able to prevent PbA-induced hepatotoxicity. The anemic effect of PbA as observed in the low percentage of PCV and hemoglobin was reversed by treatment with Trévo™, which was able to increase the percentage of PCV and Hb volume. Treatment with Trévo™ also reduced the total WBC and other blood parameters such as neutrophil, lymphocyte, monocyte, eosinophil, and basophil. A decrease in Hb content by PbA has been linked to the ability of PbA to inhibit erythropoiesis or its binding to RBC, which often results in low Hb content and short life for RBC respectively. Another anemic effect of PbA can also be due to its displacement of Fe from Hb or inhibiting the activity of aminolevulinic acid dehydratase (ALAD), all culminating in the reduction of Hb and lifespan of RBCs. The anemic effect of toxic heavy metals has been reported by various investigators and all supported our findings [ 28– 32]. PbA administration for 10 days caused a significant increase in serum levels of AST ad ALT. The increased level of these enzymes might be due to leakage from the tissue as a result of damage caused by PbA. This observation is similar to the report of [ 2, 3]. They reported that elevated serum levels of ALT and AST can be linked to damage of the membrane due to oxidative stress induced by PbA. This increased the permeability of the enzymes from the hepatocytes into blood circulation. In addition, PbA exposure also caused a significant decrease in serum albumin. Albumin plays an important role in blood detoxification. The depletion of albumin can further enhance the toxicity of PbA. One of the ways by which PbA caused albumin reduction is through forming a complex with the protein, thereby making it lose its biological function [ 33, 34]. Pretreatment with Trévo™ before concurrent administration of PbA caused a significant reduction in the serum level of AST ad ALT and increase concentration of albumin. The hepatoprotective effect of Trévo™ against cyanide ad acetaminophen-induced liver injury has been reported [ 21, 22]. Trévo ameliorated the hepatotoxicity of PbA by preventing damage to the hepatic membrane and leakage of the enzyme into the blood. In addition, it is possible that Trévo™ chelate PbA, thus preventing it from binding to a functional molecule such as albumin. Our result also supported the oxidative stress-induced mechanism of PbA as reported by other researchers [ 2– 4, 16, 17]. PbA exposure caused a mild increase in MDA level as well as a significant decrease in GSH concentration, CAT, SOD, and GST activities in liver tissue. The mechanism of PbA-induced oxidative stress involves, might involve increased production of ROS as a result of damage to enzymes involved in mitochondria respiration. The ROS generated react with biomolecules increasing peroxide products such as lipid peroxide ad protein carbonyl, further stressing the antioxidant system, as observed in the low level of CAT, SOD, and GST. The histopathological examination of the liver tissue in rats administered PbA corroborates the findings of the biochemical assays. Morphological changes observed include congestion of the central venules, hepatic necrosis, and abscess in the liver parenchyma decreased portal triads and necrotized hepatocytes. Pretreatment with Trévo™ prevents the morphological changes observed. Some of the phytochemicals present in Trévo™ have been reported to improve liver functions and the purification of the organ. Pomegranate, Amalaki, and Phyllanthus emblica [ 36, 37]. These are compounds with established antioxidant and hepatoprotective properties. Therefore, the ability of Trévo™ to prevent enzyme leakages and the oxidative effect of PbA might not be unconnected with the phytochemicals present in the product.
Conclusions
In conclusion, our results show that Trévo™ was hepatoprotective against lead toxicity and the activity might be linked to the presence of numerous antioxidants phytochemicals present in Trévo™.
Acknowledgement
The authors will appreciate some of the students who assisted in conducting the experiment.
Conflict of interest
The authors declare no competing interest.
Notes
CRediT author statement
OBI: Conceptualization, Methodology, Investigation, Data Curation, Writing-Original draft preparation, EFA: Writing-Review & Editing, Formal analysis; TTO: Conceptualization, Data curation; BC: Resources
References
1. Bischoff K, Mukai M, Ramaiah SK. Liver toxicity. Veterinary Toxicology. Academic Press Elsevier; Amsterdam, The Netherlands: 2018. p. 239-257.  2. Al-Megrin WA, Alkhuriji AF, Yousef AOS, Metwally DM, Habotta OA, Kassab RB, et al. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants 2019;9(1):10
https://doi.org/10.3390/antiox9010010
.    3. Alhusaini A, Fadda L, Hasan IH, Zakaria E, Alenazi AM, Mahmoud AM. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating Akt/GSK-3 β Signaling Pathway. Biomolecules 2019;9(11):703
https://doi.org/10.3390/biom9110703
.    4. Abdelhamid FM, Mahgoub HA, Ateya AI. Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environmental Science and Pollution Research 2020;27(10):10950-10965
https://doi.org/10.1007/s11356-020-07718-3
.   7. Al-Dbass AM, Al-Daihan SK, Bhat RS. Agaricus blazei Murill as an efficient hepatoprotective and antioxidant agent against CCl4-induced liver injury in rats. Saudi Journal of Biological Sciences 2012;19(3):303-309
https://doi.org/10.1016/j.sjbs.2012.03.004
.    10. Mujaibel LM, Kilarkaje N. Mitogen-activated protein kinase signaling and its association with oxidative stress and apoptosis in lead-exposed hepatocytes. Environ Toxicol 2015;30(5):513-529
https://doi.org/10.1002/tox.21928
.   11. Liu J, Lewis G. Environmental toxicity and poor cognitive outcomes in children and adults. J Environ Health 2014;76(6):130.   12. Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res 2014;21(18):11066-11074
https://doi.org/10.1007/s11356-014-2986-5
.   13. Abdel Moneim AE. Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res 2012;148(3):363-370
https://doi.org/10.1007/s12011-012-9370-4
.   14. Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 2016;23(9):8244-8259
https://doi.org/10.1007/s11356-016-6333-x
.   15. Mudipalli A. Lead hepatotoxicity & potential health effects. Indian J Med Res 2007;126(6):518.  16. Adli DEH, Brahmi M, Kahloula K, Arabi W, Bouzouira B, Talatizi M, et al. The therapeutic effect of Cinnamomum cassia essential oil against hepatotoxicity induced by co-exposure to lead and manganese in developing Wistar rats. Journal of Drug Delivery and Therapeutics 2020;10(5):49-55.  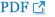 17. Chen C, Lin B, Qi S, He J, Zheng H. Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley Rats. Biological Trace Element Research 2019;191(2):426-434
https://doi.org/10.1007/s12011-019-1635-8
.   18. Flora SJ, Flora G, Saxena G. Environmental occurrence, health effects and management of lead poisoning In Lead Chemistry, Analytical Aspects. Environmental Impacts and Health Effects. In: Cascas SB, Sordo J, editors. Elsevier Publication; Amsterdam, The Netherlands: 2006. p. 158-228
https://doi.org/10.1016/B978-044452945-9/50004-X
.  19. Ilesanmi OB, Ikpesu T. Neuromodulatory activity of trèvo on cyanide-induced neurotoxicity viz neurochemical, antioxidants, cytochrome C oxidase and p53. Advances in Traditional Medicine 2021;21(2):297-304
https://doi.org/10.1007/s13596-020-00450-w
.  20. Ilesanmi OB, Atanu FO, Odewale TT, Adeogun E, Chikere BN, Alaneme CU, et al. Effect of a phytonutrient-rich product and administration time on cyanide-induced cardiotoxicity. Trop J Nat Prod Res 2020;4(7):304-309.  21. Akinmoladun AC, Oguntunde KO, Owolabi LO, Ilesanmi OB, Ogundele JO, Olaleye MT, et al. Reversal of acetaminophen-generated oxidative stress and concomitant hepatotoxicity by a phytopharmaceutical product. Food Science and Human Wellness 2017;6(1):20-27
https://doi.org/10.1016/j.fshw.2016.11.001
.  22. Babatunde IO, Abigail A. Ameliorative effect of a multi-nutrient-rich product against cyanide induced hepatorenotoxicity. Drug Discovery 2021;15(35):34-42.
24. Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 1973;187(1):195-202.  28. Dewanjee S, Sahu R, Karmakar S, Gangopadhyay M. Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem Toxico 2013;55: 78-91
https://doi.org/10.1016/j.fct.2012.12.040
.   29. Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, et al. Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem Toxicol 2014;65: 260-268
https://doi.org/10.1016/j.fct.2013.12.041
.   30. Abdel-Moneim AM, El-Toweissy MY, Ali AM, Awad Allah AAM, Darwish HS, Sadek IA. Curcumin ameliorates Lead (Pb 2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biological trace element research 2015;168(1):206-220
https://doi.org/10.1007/s12011-015-0360-1
.   31. El-Boshy ME, Refaat B, Qasem AH, Khan A, Ghaith M, Almasmoum H, et al. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in a rats. Environmental Science and Pollution Research 2019;26(22):22736-22746
https://doi.org/10.1007/s11356-019-05562-8
.   33. Mahaldar K, Hossain A, Islam F, Islam S, Islam MA, Shahriar M, et al. Antioxidant and hepatoprotective activity of Piperretro fractum against Paracetamol-induced hepatotoxicity in Sprague-Dawley rat. Natural Product Research 2020;34(22):3219-3225
https://doi.org/10.1080/14786419.2018.1550768
.   34. Ahmed RA. Hepatoprotective and antiapoptotic role of aged black garlic against hepatotoxicity induced by cyclophosphamide. The Journal of Basic and Applied Zoology 2018;79(1):1-8
https://doi.org/10.1186/s41936-018-0017-7
.  35. Huang CZ, Tung YT, Hsia SM, Wu CH, Yen GC. The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food Funct 2017;8(2):842-850
https://doi.org/10.1039/C6FO01585A
.  36. Kumar V, Kshemada K, Ajith KG, Binil RS, Deora N, Sanjay G, et al. Amalaki rasayana, a traditional Indian drug enhances cardiac mitochondrial and contractile functions and improves cardiac function in rats with hypertrophy. Sci Rep 2017;7(1):1-17
https://doi.org/10.1038/s41598-017-09225-x
.    37. Husain H, Latief U, Ahmad R. Pomegranate action in curbing the incidence of liver injury triggered by Diethylnitrosamine by declining oxidative stress via Nrf2 and NFκB regulation. Sci Rep 2018;8(1):1-17
https://doi.org/10.1038/s41598-018-26611-1
.   
Figure 1
The concentration of lipid peroxides product - malondialdehyde (MDA), in the liver tissue of male rats after 2 day-pretreatment with Trévo™ and 12-day co-administration with lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. 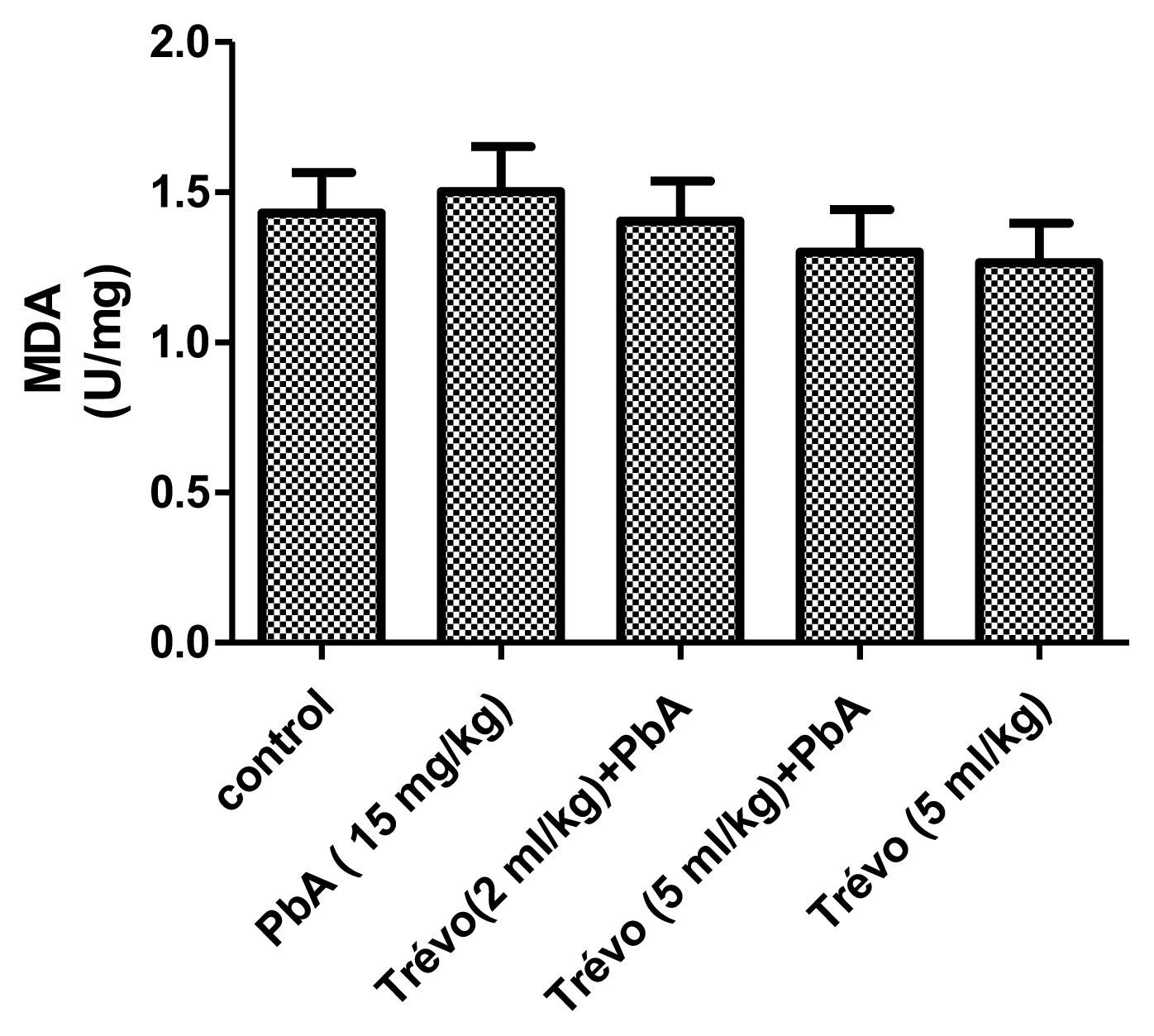
Figure 2
The concentration of non-enzymatic antioxidant-reduced glutathione (GSH) in the liver tissue of male rats after 2 day-pretreatment with Trévo™ and 12-day co-administration with lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; #P<0.05= PbA vs. Trévo™ (5 mL/kg) + PbA 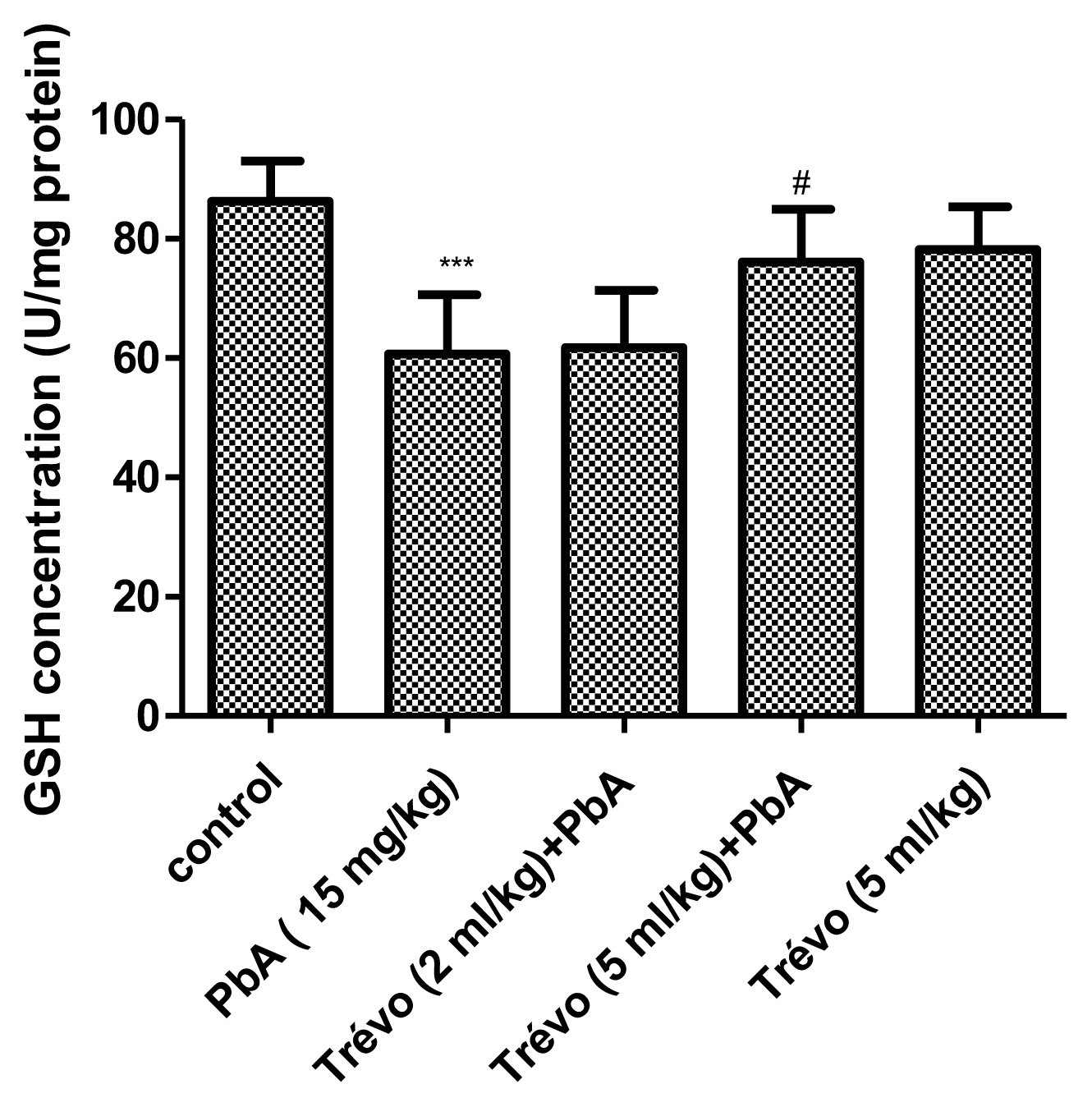
Figure 3
The catalase activity in the liver tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; ###P<0.001= PbA vs. treatment groups 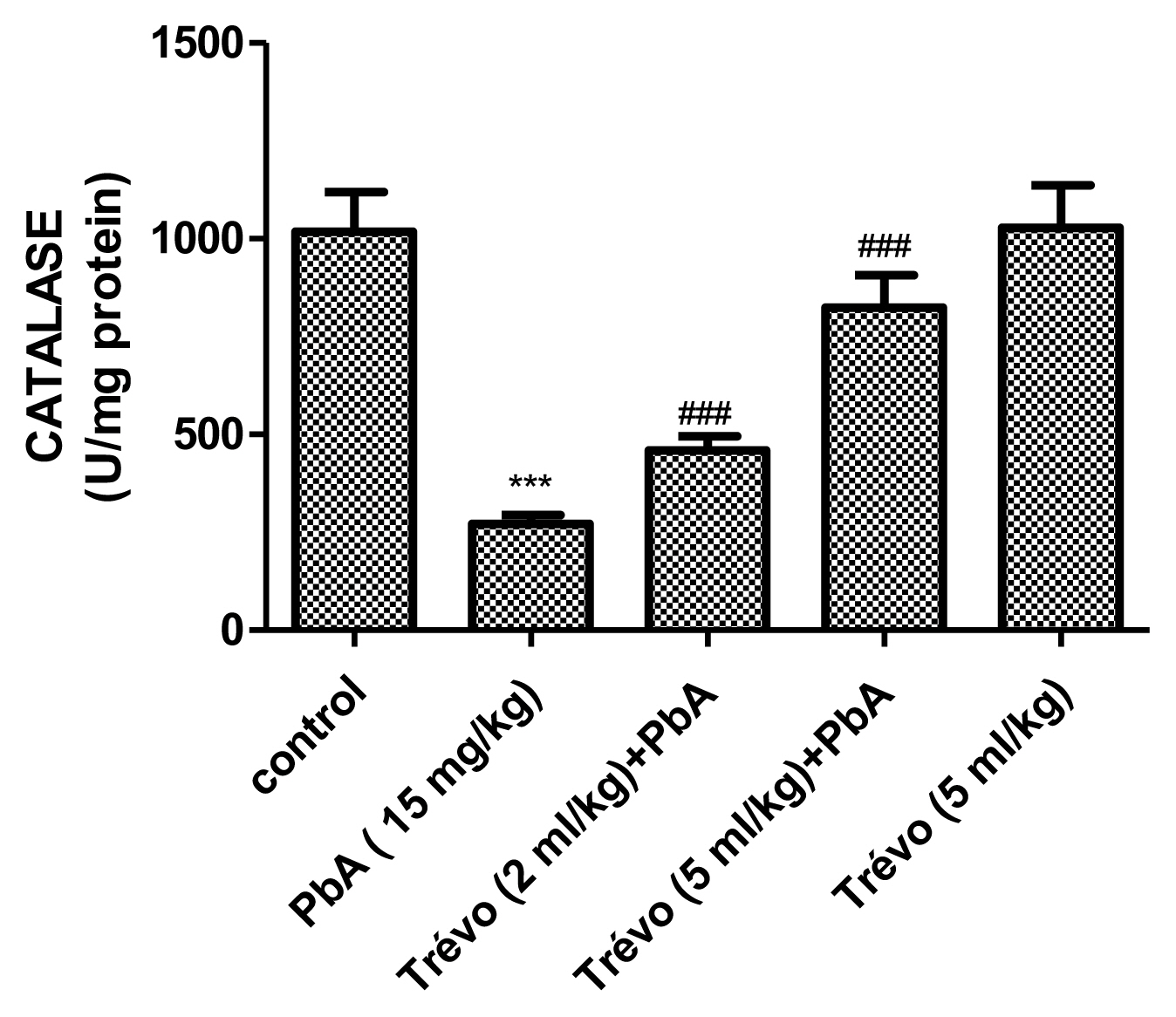
Figure 4
The superoxide dismutase (SOD) activity in the kidney tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/Kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; #P<0.05= PbA vs. Trévo™ (2 mL/kg) + PbA; ##P<0.01= PbA vs. Trévo™ (5 mL/kg) + PbA 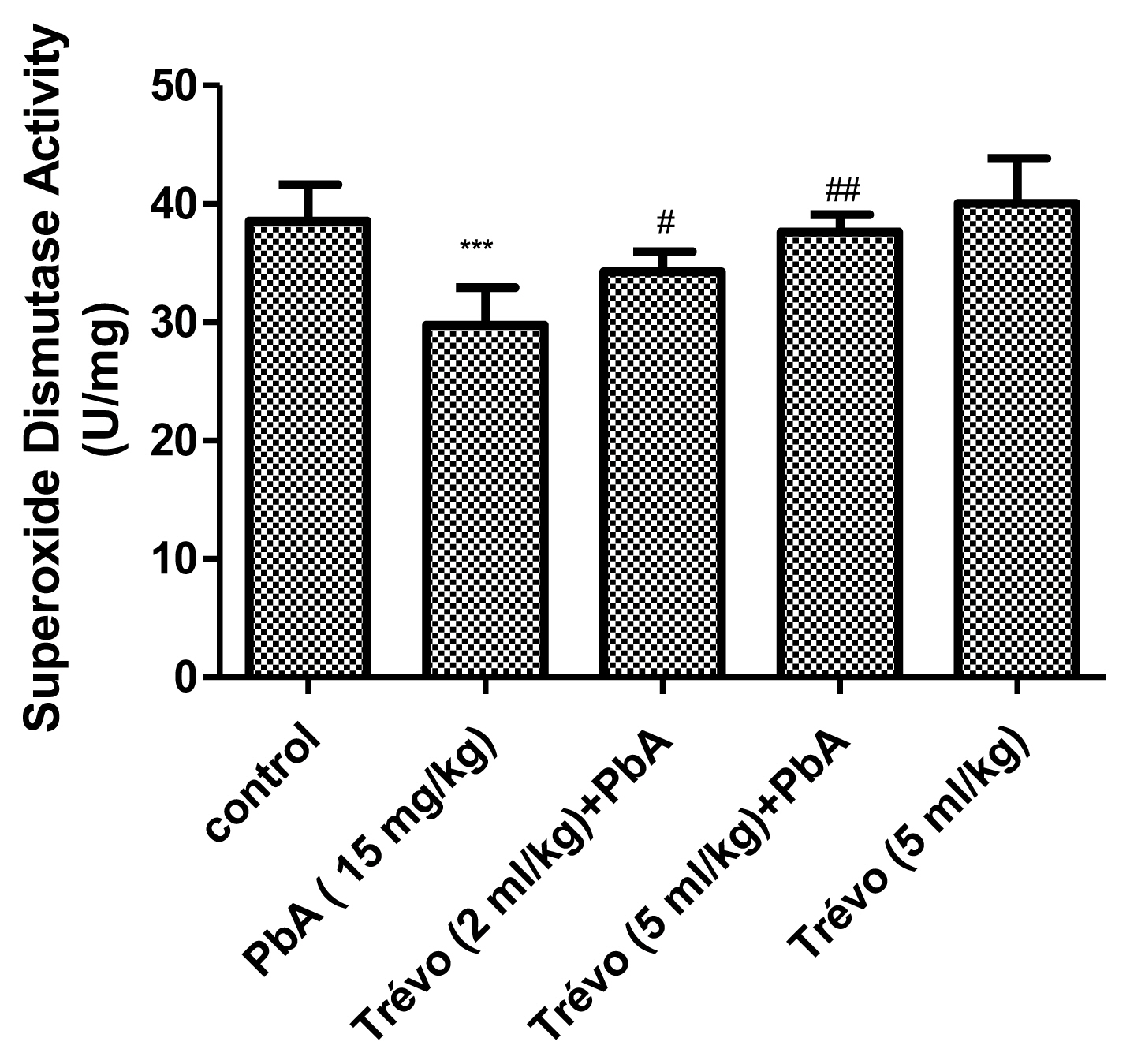
Figure 5
The glutathione-S-transferase (GST) activity in the liver tissue of male rats after 7 day-pretreatment with Trévo™ and 12-day concomitant exposure to lead acetate (15 mg/kg) via intraperitoneal administration. Data are shown as mean±standard deviation (SD) for 7 animals. Statistically significant differences: ***P<0.001= control vs. PbA; ##P<0.01= PbA vs. Trévo™ (2 mL/kg) + PbA; ###P<0.001= PbA vs. Trévo™ (5 mL/kg) + PbA 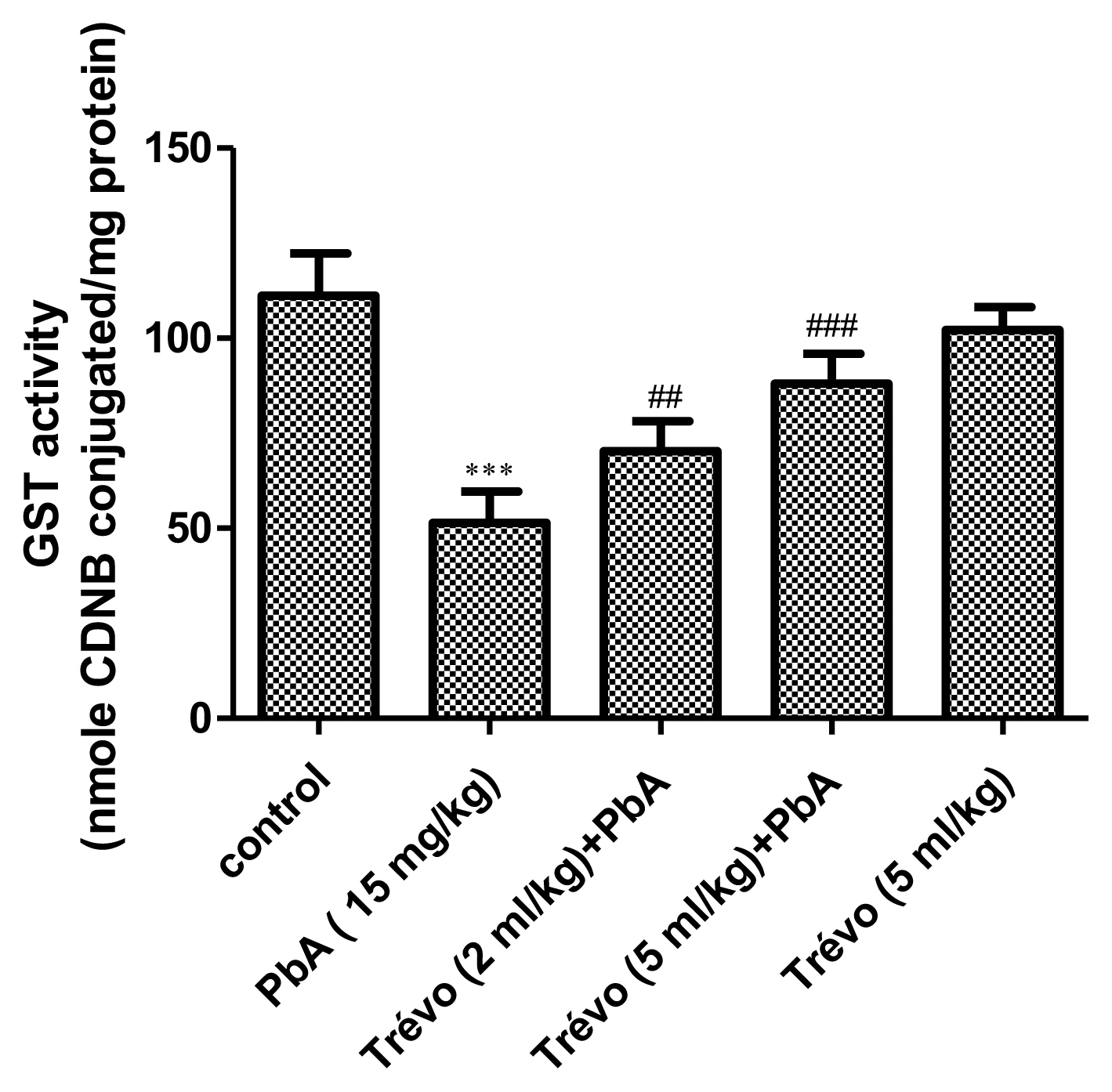
Figure 6
Photomicrograph of rat liver sections stained with hematoxylin and eosin (X100) showing alterations in the liver after treatment with trévo and exposure to PbA. The blue arrow indicates congestion. The green arrow indicates restoration and normal architecture of hepatic cords. The red arrows indicate normal hepatic artery and portal vein. 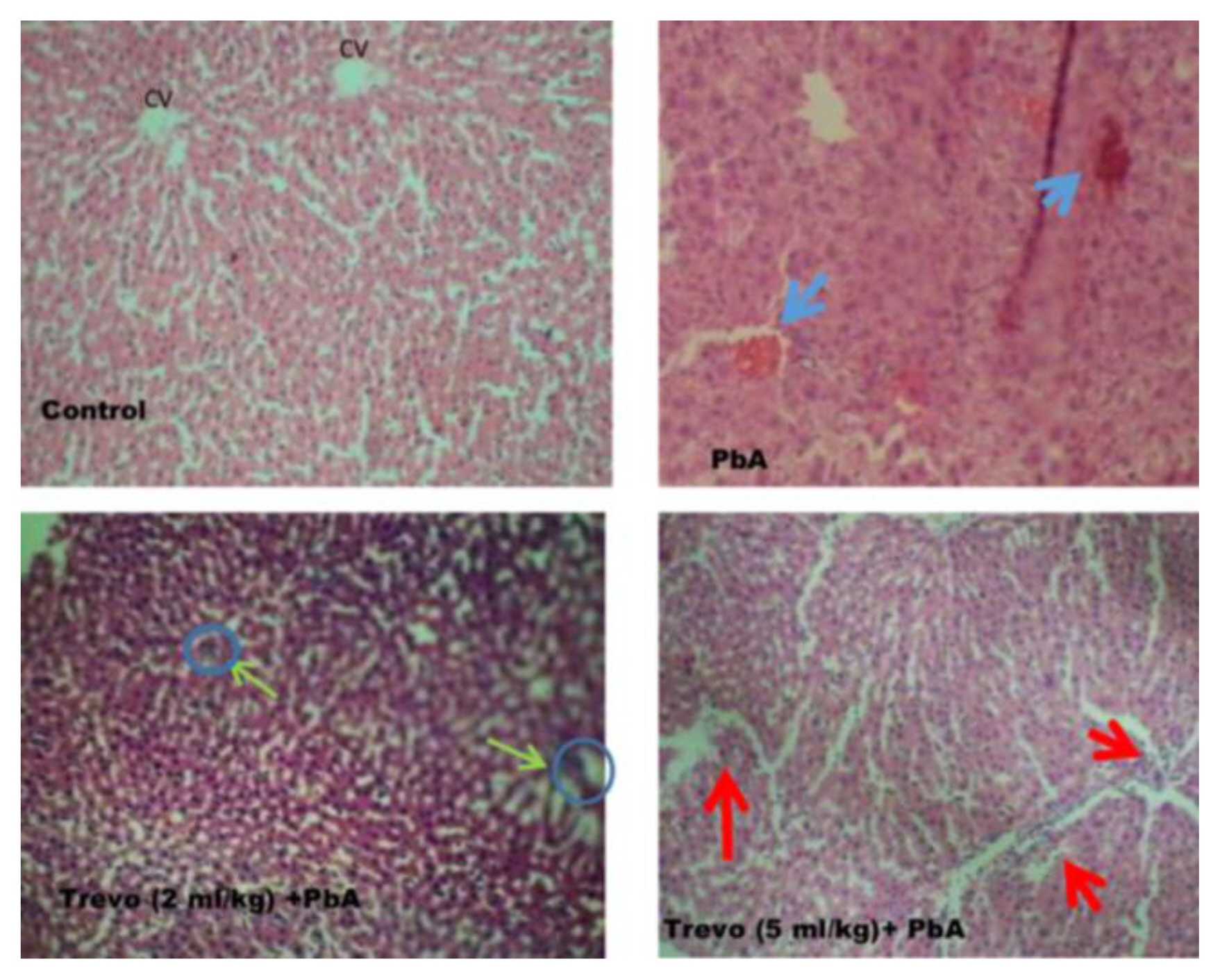
Table 1
The effect of Trévo™ on blood hematology of rats exposed to PbA.
|
Group |
PCV (%) |
HB (g/dL) |
TOTAL WBC count (g/dL) |
NEUT (%) |
LYM (%) |
MONO (×109L) |
EOSINO (×109L) |
BASO (×109L) |
|
Control
|
44.7±5.77 |
14.9±1.54 |
5.4±0.10 |
49.7±2.63 |
35.5±3.98 |
5.9±0.69 |
2.3±0.15 |
0.2±0.01 |
|
PbA
|
29.7±3.07*
|
10.2±1.67*
|
9.0±1.05*
|
55.3±5.96*
|
47.2±3.19*
|
7.4±0.51*
|
3.7±0.03*
|
0.6±0.04*
|
|
2 mL/kg Trévo™ +PbA
|
42.3±4.46#
|
14.1±1.49#
|
5.9±0.79#
|
50.0±4.54#
|
39.3±4.04#
|
7.0±0.89 |
3.0±0.08#
|
0.3±0.03#
|
|
5 mL/kg Trévo™ +PbA
|
43.7±3.89#
|
14.6±1.16#
|
8.2±0.52#
|
51.4±4.30#
|
40.9±4.50#
|
6.7±0.89#
|
3.0±0.14#
|
0.0±0.00#
|
|
5 mL/kg Trévo™ |
42.3±3.72 |
14.1±1.26 |
5.5±0.62 |
48.0±5.06 |
30.0±3.68 |
5.7±0.60 |
2.0±0.02 |
0.0±0.00 |
Table 2
Serum ALT and AST activities and total albumin levels in control and in all experimental groups.
|
Groups |
AST (IU) |
ALT (IU) |
ALB (mg/dL) |
|
Control
|
13.6±1.51 |
8.5±0.97 |
7.0±0.33 |
|
PbA
|
19.5±2.07***
|
14.5±1.56***
|
3.6±0.26***
|
|
2 mL/kg Trévo™ +PbA
|
18.0±1.10 |
8.1±0.52###
|
5.6±0.62###
|
|
5 mL/kg Trévo™ +PbA
|
17.7±1.10 |
11.5±1.14###
|
6.0±0.59###
|
|
5mL/kg Trévo™ |
13.4±1.21 |
8.0±0.53 |
7.1±0.30 |
|
|


















