Determination of pesticide residue content in fruits and vegetables from Lagos, Nigeria
Article information
Abstract
Developing countries like Nigeria are increasingly employing pesticides to boost the productivity of their agriculture and food supply, despite the fact that doing so poses a health risk to the general populace. The purpose of this study was to assess pesticide residue levels in Lagos, Nigeria. A total of 18 samples from three neighbourhood markets were collected, and they were then examined for the presence of organochlorine (Endosulfan I, Pentachlorophenol, Heptachlor epoxide, and Endosulfan II) and organophosphate (Dichlorvos, Dimethoate, Phorate, and methyl parathion) residues. During the pre-treatment, the multi-residue Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS citrate) method with the addition of acetonitrile was used, and then samples were analysed using GC-MS. All of the samples contained dichlorvos, but the orange sample's concentration was below the limit of quantification, making quantification impossible. Dimethoate concentrations were below MRLs except waterleaf sample. With a concentration of 0.043 μg/mL, Waterleaf had the highest quantity of dimethoate in the sample. The findings of this study indicate that in order to safeguard the health of consumers, it is necessary to closely monitor organochlorine and organophosphate use nationwide, along with other related pesticides, and to test for pesticide residues in food products.
Introduction
Fruits and vegetables are essential components of a healthy diet. Consuming a variety of fruits and vegetables has numerous benefits, including protection against type 2 diabetes and some cancers; it aids in the reduction of obesity, lowers cholesterol and blood pressure (hypertension); and it is a source of essential minerals and vitamins such as vitamin C, E, and A, magnesium, zinc, and potassium, which are required for the majority of bodily reactions [1]. Pests and illnesses harm fruits and vegetables, as well as other crops, during production and storage, resulting in losses that reduce product quality and yield. Fruits and vegetables, like other crops, are subject to pests and sickness, which can cause damage during production and storage, lowering crop quality and yield [1].
Plant pests and diseases, in general, cause considerable crop losses, encouraging the use of pesticides to boost farm output. One of the primary goals of modern agriculture is to increase plant production while safeguarding the environment [2]. Pesticides, on the other hand, are xenobiotics; their frequent use during production leaves pesticide residues in crops after harvest; as a result, it is critical to effectively focus on monitoring and regulating their use from public health and environmental sustainability perspectives [3]. Day by day, more is understood about the harm that agricultural chemicals used for plant protection and nourishment do to the environment and to human health [4]. The primary problems are their toxic effects, which include the potential to harm prenatal development and the reproductive systems as well as to cause cancer and asthma [5,6]. Due to the persistence of the majority of pesticides, prolonged exposure arises from their ongoing presence in the body. Organochlorine (OCP) and organophosphate (OP) residues can harm developing children's DNA, endocrine system, and nerve and brain cells [7].
Despite the risk to the public’s health, developing countries like Nigeria are increasingly applying these chemicals to agricultural products to boost food and agricultural productivity [8]. These chemicals are applied to manage the rising demand for food while preventing crop harm. The Nigerian government, however, does not adequately monitor the situation and lacks internal norms, and controls [7]. The WHO reports around 3 million cases of pesticide poisoning each year, leading to 220,000 fatalities worldwide [9]. To meet the tremendous demand for food in Nigeria, a developing nation with a population of over 200 million, agricultural products are mass-produced using pesticides. To prevent crop damage and provide the huge demand for food in the country, these pesticides are utilized. Lagos is the largest city in Africa and one of the metropolises with the highest growth rates globally, with a population of over 20 million. It is the city with the biggest concentration of industry and the hub of much of the nation's wealth and economic activities. Due to its industrialized character and big population, Lagos obtains the majority of the food produced in the northern area, other regions of Nigeria and neighboring countries.
Every day we are exposed to environmental pollutants, and food products are no exception. Pesticides are pollutants that typically show up in food production when there are pesticide-using agricultural activities going on. Pesticide residues can still be discovered in crops even after harvest because of the persistence of pesticides, and the concentration of these pesticides has become a problem because it causes various health problems when consumed. The six various pesticides that were tested in the samples were selected based on their accessibility, frequency of use, and class (organochlorine and organophosphate), as the active compounds in these classes are more toxic and harmful to the environment. In order to evaluate and contrast the results with the legal limitations, this study monitors pesticide residues and their amounts in fruits and vegetables sold at major fresh produce markets in Lagos.
Materials and Methods
Study area
The location of this study was Lagos, Nigeria, with coordinates 6.455027oN 3.384082oE. With a population of over 20 million and an area of 1,171.28 km2, Lagos is the largest metro city in Africa, a significant financial hub for the continent, and the foundation of both Lagos state and Nigeria's economies. It has twenty local governments and local markets are distributed all over the local governments from which samples were collected. Due to Lagos large population, it receives the majority of the food produced in the northern region and other regions of Nigeria due to its industrialized nature and sizable population.
Sample collection
In this study, eighteen samples (triplicate per sample) were collected from three major fruit and vegetable markets within Lagos. The markets include Agboju market, Ketu fruit market, and Mile 12 market, which is the biggest foodstuff market in the study area. Collected samples included fruits and vegetables (orange, banana, cucumber, pumpkin leaf, waterleaf, and tomato) samples. Samples were randomly collected from various fruit markets from May 2022 to June 2022. Polythene bags were used to collect the samples, and each bag was labelled with an identification code, location, time, and date of the sampling before they were transported to the University of Lagos Analytical and Environmental Laboratory and stored at -20 °C.
Chemicals and reagents
OCP and OP standards used were obtained from AccuStandard. Acetonitrile and Trisodium citrate dehydrate were bought from Qualikems. Graphitized Carbon Black sorbent (GCB) was obtained from supelcoSupelclean Envi-Carb. D-SPE 15 ml tubes (CEN method 15662), Citrate buffer tube (CEN method 15662), and ceramic homogenizers were obtained from waters corporation.
Sample preparation and pre-treatment
Thirty-six pesticide residues were screened in the produce and prepared samples. The samples were prepared, cleaned and extracted using the modified Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS citrate) approach for the determination of pesticide residues [10]. Two grams of laboratory samples were chopped before they were blended for homogenization. Dried ice was added during the homogenization process to enhance the breaking ability of the samples thereby achieving better homogeneity. Ten grams of water was added to a 50 ml centrifuge containing 10 g of the homogenized sample. This was done to optimize the extraction. Ceramic homogenizers were added to the tubes as well to help reduce the shaking time and ensure there is no loss in pesticide recovery. 10g of the homogenous sample was weighed into a 50 ml centrifuge tube and shaken for 1min using an orbital scilogex shaker at a speed of 300 rpm. An 8 ml aliquot of the extract was put into a PP-single-use centrifugation tube together with 1.2 g of magnesium sulphate (MgSO4) and 200 mg of primary secondary amine (PSA) per mL of extract. Some samples included the addition of graphitized carbon black (GCB), which is crucial for the efficient removal of pigments like chlorophyll. The tubes were shaken vigorously for 2 mins (because of the GCB) and centrifuged for 5 mins at 3,500 rpm. Clean extracts were transferred into a capped vial and then filled into vials and injected into GC-MS for analysis.
Validation
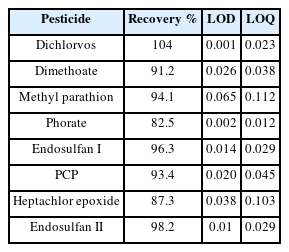
Several measures were used throughout the study to assure quality control of results, beginning with sampling and progressing through sample homogenization, extraction, instrumental analysis, and data interpretation. The various measures included the use of a validated sampling protocol that guided how representative samples from the field were to be picked, how systematic labelling had to be done, and how the sampled samples were to be transported from the field to the laboratory. During data presentation, quality control of the analysis of the tomato matrix was done to verify the accuracy, linearity, Limit of detection (LOD), Limit of Quantification (LOQ), and recovery of the analytical techniques used to prepare the sample and analyze it. According to the SANTE recommendations (SANTE/12682/2019 2020), the validation of the analytical procedures was done. By examining the linearity, recovery, precision, and specificity of peak regions, the approach was found to be reliable. The LOD and LOQ, respectively, as well as the signal accepted at 3 and 10 times the noise ratio, served to confirm the sensitivity of the approach. By graphing the area of each target pesticide against the concentrations of the calibration standards (calibration curves in tomato), the matrix-matched calibrations for the linearity test were established. With correlation coefficients ranging from 0.9922-0.9959, good linearity was obtained in each case. According to the SANTE recommendations (SANTE/12682/2019 2020), mean recoveries from the initial validation should be between 70 % and 120 % for all analytes within the scope of a method, with an associated repeatability RSD ≤ 20% for precision. It was discovered that pesticide recoveries ranged between 82 % and 104 %. Overall, the average recovery, SD, and RSD values were discovered to be 93.4%, 6.6, and 7.07%, respectively. The analyzed pesticides had LODs between 0.001µ g ml-1 and 0.038µ g ml-1and LOQs between 0.012 and 0.112 µ g ml-1. The method is regarded as valid because the results for all pesticides discovered in the fruit and vegetable samples from this study were within the established acceptability limits (7 0% Q 120 % and RSD 20 %).
GC-MS Analysis
All extracts were analyzed using GC-MS (GC – Agilent 7890 and MS detector – Agilent 5975). The GC’s initial temperature was 80°C, which was held for 8minutes and then increased to 340 °C at a rate of 17 °C min-1, Injection vials of 1.5 mL vials for GC-autosampler were used. Analytes were separated with a DB-5MS UI column (30 m x 0.25 mm x 0.25 µ m). Helium was used as the carrier gas and the total run time was 24 minutes. All of the organophosphates were eluted within a retention period, around 10.9 minutes, and were resolved under the optimized GC conditions. Table 1 summarizes the limit of detection and limit of quantification of each pesticide.
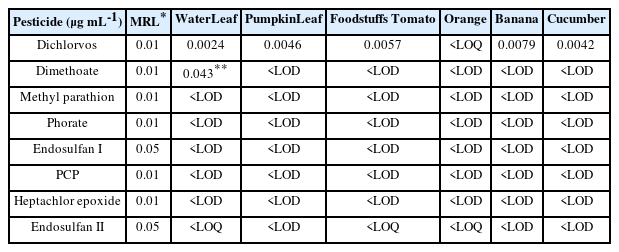
The pesticides identified in food samples are listed in Table 2. Dichlorvos was present in all of the samples, however the orange sample's amount was below the limit of quantification, making it impossible to quantify. Except for the dimethoate found in the waterleaf sample, all examined samples had concentrations below their Maximum Residue Levels (MRLs). Additionally, waterleaf had the highest concentration of dimethoate from the study, with a concentration of 0.043 µg mL-1.
Discussion
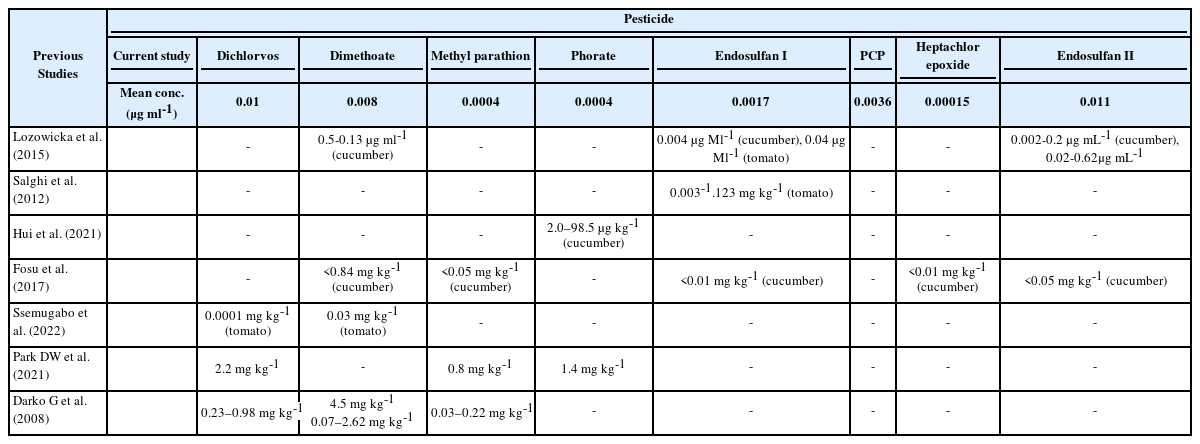
To improve the quality of food products and crop yields, pesticide application is a popular practice around the world. Pesticides may have detrimental consequences on both human and environmental health, despite their many benefits. As a result, the use of pesticides is carefully regulated in developed economies. However, these management and monitoring procedures are generally absent in developing nations. In Nigeria, a developing country with a population of over 200 million, agricultural products are mass-produced using pesticides to supply the enormous demand for food. These pesticides are used to prevent crop damage and meet the enormous demand for food in the nation. In this study, six different foods—three of each—were gathered from local markets and evaluated using GC-MS for the presence of dichlorvos, Dimethoate, methyl parathion, phorate, endosulfan I, endosulfan II, PCP, and heptachlor epoxide. Dichlorvos was present in all foods except orange samples, per the study's findings, however it did not surpass the MRL limit. Waterleaf samples contained amounts of Dimethoate that were over the allowable level. In contrast to this study, Oyeyiola et al. (2017) discovered heptachlor epoxide to be less than 0.000050 µg ml-1 in cucumber and Endosulfan I to be present in tomatoes at a concentration of 0.00016 µg ml-1 [7]
As shown in Table 3, the study by Lozowicka et al., (2015) Endosulfan I, Endosulfan II and Dimethoate had a concentration in the range of 0.004-0.04 µg ml-1, 0.002-0.2 µg ml1 and 0.5-0.13 µg ml-1 respectively in cucumber samples from Kazakhstan. From the same study, tomato samples also contained Endosulfan I and II in the range of 0.030-0.120 µg ml-1 and 0.020-0.620 µg ml-1 all of which have a higher concentration compared to that of this study [12]. Salghi et al., (2012) ascertained the presence of pesticide residues in tomatoes in Morocco and found Endosulfan to be present in all the samples in the range of 0.003 to 1.123 mg kg-1. Only one among all the analyzed samples was below the ADI for Endosulfan [13].
In another study, Hui et al., (2021) determined the presence of Phorate in cucumber with the range of 2.0–98.5 µg kg-1 in China [14]. In Ghana, pesticide residues were found to be present in fruits and vegetables. In cucumber Heptachlor, Endosulfan I, Endosulfan II, Dimethoate, Parathion had a concentration of < 0.01 mg kg-1, < 0.01 mg kg-1, < 0.05 mg kg-1, < 0.84 mg kg-1 and < 0.05 mg kg-1 respectively. In Tomatoes Dimethoate was found to have a concentration < 0.03 mg kg-1 [15]. A current study in the Kampala area of Uganda determined the presence of pesticides in fruits and vegetables. Among the pesticides present are Dichlorvos and Dimethoate. Dichlorvos was present in every sample with a mean concentration of 0.0001mg kg-1 in tomatoes, which is lower than that in this study [16].
30 % of the samples analyzed contained pesticide residues above MRL with Chlorpyrifos being the common pesticide found in the samples [17]. In South Korea, the presence of pesticide residues in leafy vegetables mostly in the form of seasoned vegetables in a 15-year study on pesticide residue was reported. Among the pesticides found to be present are Endosulfan, Dimethoate and Dichlorvos. Endosulfan was reported to be above MRLs in 6.6 % of samples containing Endosulfan. Endosulfan was found in different vegetable samples in the range of 0.20 mg kg-1 – 6.20 mg kg-1 which is higher than that of this study [18]. Also, Yu et al., in their study reported the presence of Dimethoate, Dichlorvos, Phorate and methyl parathion among others in vegetables from China. 84.3 % of pesticides were found present at levels that exceeded MRLs and 40.7 % of these pesticides are among the list of pesticides banned for agricultural use in the country. Dichlorvos and Dimethoate were reported to be among the pesticides having most commonly detected pesticides with a percentage up to 8.7% and 8.1% respectively. In the same study, maximum values of 2.2 mg kg-1, 4.5 mg kg-1, 1.4 mg kg-1, and 0.8 mg kg-1 in Dichlorvos, Dimethoate, Phorate and Methyl parathion were recorded respectively, and the values are higher than that of the present study [19].
A study by Parveen et al. in Pakistan reported the presence of pesticide residues in vegetables. Although the concentrations of pesticides detected were not stated, it was reported that up to 63% of them were contaminated with various pesticides and it was observed that 46% of the contaminated samples recorded exceeded MRLs. Endosulfan and Dimethoate were among the pesticides recorded to be present in tomato and pumpkin samples [20]. In Kumasi Ghana, most of the vegetables were reported to contain pesticide residues with Dichlorvos being the most frequently detected pesticide in the analyzed samples with a concentration in the range of 0.23 – 0.98 mg kg-1, which is higher when compared to that of the present study. Dimethoate and Methyl parathion were reportedly present in tomato samples with a concentration in the range of 0.07 – 2.62 mg kg-1 and 0.03 – 0.22 mg kg-1 which are both higher than that of this study [21].
A study by Parveen et al. in Pakistan reported the presence of pesticide residues in vegetables. Although the concentrations of pesticides detected were not stated, it was reported that up to 63% of them were contaminated with various pesticides and it was observed that 46% of the contaminated samples recorded exceeded MRLs. Endosulfan and Dimethoate were among the pesticides recorded to be present in tomato and pumpkin samples [20]. In Kumasi Ghana, most of the vegetables were reported to contain pesticide residues with Dichlorvos being the most frequently detected pesticide in the analyzed samples with a concentration in the range of 0.23 – 0.98 mg kg-1, which is higher when compared to that of the present study. Dimethoate and Methyl parathion were reportedly present in tomato samples with a concentration in the range of 0.07 – 2.62 mg kg-1 and 0.03 – 0.22 mg kg-1 which are both higher than that of this study [21].
From the findings in this study, there is a need for monitoring, risk assessment, and regulation for Dichlorvos and Dimethoate. Also, users should ensure strict compliance with all practices such as Good Agricultural Practices, Good Storage Practices, and Good Hygienic Practices among other safety practices when handling pesticide products. The country’s Agency should work on the compilation of data from researchers as these data will be a baseline on which future strategies could be implemented for the safety of the consumers and the environment. Biomonitoring of pesticides among users should be seen as a prospect for future research work in the country.
Conclusions
The pesticide residues present in the fruits and vegetables analyzed revealed that the concentrations were lower when compared to other countries. The findings from the study also revealed that all samples except Waterleaf that contained high level of Dimethoate had pesticide residues lower than their respective Maximum Residue Level. Nevertheless, there is a need for monitoring programs and more risk assessment studies of Dimethoate and other related pesticides.
Notes
The authors declare they have nothing to disclose.
CRediT author statement
TO: Writing- Original draft preparation, Investigation, Resources. KK: Writing- Original draft preparation, Writing- Review and Editing, Resources. DB: Conceptualization, Methodology, Writing- Original draft preparation, Writing- Review and Editing, Supervision. SS: Conceptualization, Methodology, Supervision.