Toxic hepatitis after exposure to humidifier disinfectant: A case series report
Article information
Abstract
Health damage from humidifier disinfectants is an unprecedented environmental health disaster. Humidifier disinfectants were used broadly in Korea from 1994 to 2011. Most studies have focused on respiratory problems because of the exposure route and primary respiratory symptoms. This overlooks the previous research results that humidifier disinfectants could move to extrapulmonary organs and induce toxic effects. Thus, the objective of this study was to examine toxic hepatitis cases developed after inhaling humidifier disinfectant. We focused on the indications of toxic hepatitis in two pediatric cases and one female adult case. All patients were exposed to humidifier disinfectants in a residential space. These disinfectants all contained polyhexamethylene guanidine (PHMG). Rapid increases in blood hepatic enzyme levels were seen. Two patients were discharged after treatment. Death occurred in one patient who was diagnosed with fulminant hepatitis of unknown cause. This human case series study supports prior knowledge that hepatotoxicity can occur by inhaling humidifier disinfectant.
Introduction
Humidifier disinfectant (HD) was first sold in Korea in 1994. The sales company advertised “no harm to the human body as a result of the toxicological test.” Approximately 600,000 disinfecting products from 20 brands were sold annually. Since 2006, many doctors have reported an increased incidence of unidentified acute respiratory distress syndrome (ARDS) in pediatric intensive care units (ICU) nationwide without identifying the cause. In April 2011, the Korea Centers for Disease Control & Prevention (KCDC) initiated an epidemiological investigation. On August 31, 2011, the KCDC recommended that the use of HD should be halted. A forced HD collection was carried out on November 11, 2011. By then, it was estimated that about 3.5 to 4 million people (8% of the general population) were exposed to HD in Korea [1,2].
Polyhexamethylene guanidine (PHMG) is a raw material used for HD. Health incidents caused by PHMG were reported previously. PHMG is known to inhibit dehydrogenase activity and damage bacterial cell membranes, resulting in a bactericidal effect.
PHMG aerosol is inhaled from humidifier discharge. Most patients had severe respiratory problems called humidifier disinfectant lung injury (HDLI) and many had urgent clinical symptoms. Approximately 99.5% of the respondents in a previous study complained of respiratory diseases [1]. The diseases most commonly reported were pneumonia, acute bronchitis, asthma, bronchiectasis, interstitial pneumonia, and other pulmonary diseases. These conditions were more severe in pediatric patients [3]. Previous damage analyses focused on respiratory problems. However, during a medical record review, we found several suspicious cases of toxic hepatitis. This article explores hepatotoxicity by inhalation exposure, a less recognized health effect of HD
Methods
We reviewed the medical records of patients who were diagnosed with hepatitis after exposure to HD in National Institute of Environmental Research (NIER) data. Among them, patients whose causes of hepatitis were identified or where a chronological relationship between exposure and onset could not be established were excluded first. The medical records of the remaining potential patients were reviewed in depth, and cases with clinically suspected toxic hepatitis caused by HD were selected.
There were many cases of acute hepatitis or elevated hepatic enzymes in the reviewed cases. Among the patients with hepatitis caused by HD, we included cases based on the following criteria:
-
A person who exposure to HD was confirmed through any of the following:
Any objective evidence, such as remaining product or receipts, photos, household account books, and other past records.
Specific statements about where to buy HD, when to buy it, and the product type that were confirmed through an interview.
-
Patients who had not been diagnosed with toxic hepatitis (K71) or hepatitis that was difficult to explain by other causes (K72, K73, K74, K75) before exposure to HD.
After exposure to HD and within two years after the end of HD exposure, toxic hepatitis (K71) or unspecified hepatitis (K72, K73, K74, K75) was diagnosed in a patient in the National Health Insurance Corporation data, and the patient received treatment or hospitalization, or died.
Exclusion criteria
This should not be included in the list of inclusion criteria: Cases with a clear cause of toxic hepatitis, such as viral hepatitis, drugs, or occupational toxic hepatitis. Many cases were excluded because the patients had used steroids to treat their respiratory symptoms, consumed alcohol, or were carriers of the hepatitis B virus (HBV). The three patients presented in this case series had no major causative factors of hepatitis (Table 1)
Case Description
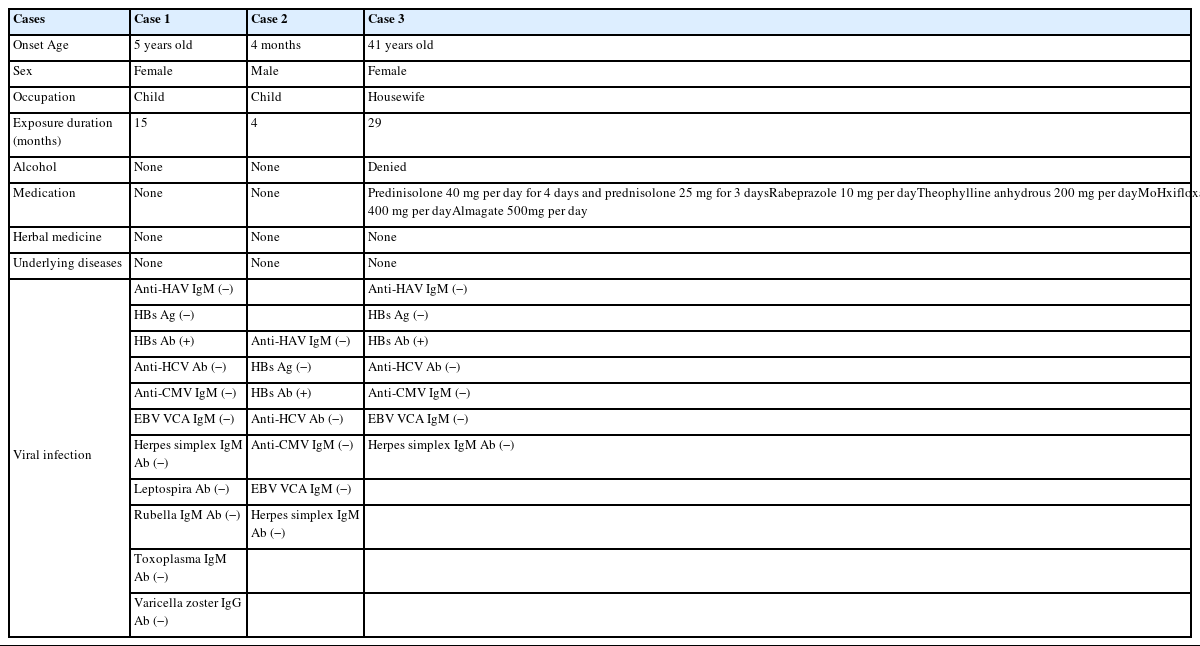
Case 1
Case 1 was a five-year-old female without any underlying diseases. The patient was born via cesarean section with a birth weight of 4.00 kg. “Findings during pregnancy and birth were normal. The child was exposed to HD for one year and three months (January 2002 – April 2003). During about 90% of the total exposure period, she was exposed to PHMG as an HD ingredient, and the remaining 10% was to chloromethylisothiazolinone/methylisothiazolinone (CMIT/MIT). Her parents stated that the humidifier was used 24 hours a day and the distance between the humidifier and the patient was less than 0.5 meters.
The patient went to the hospital due to fever, general weakness, epigastric pain, anorexia, and nausea. Symptoms had begun three days earlier. The patient had a confirmed body temperature over 39°C and hepatomegaly. At admission, her aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were confirmed to be 268 and 118 units/L, respectively (Fig. 1). Her hepatic enzyme levels increased to 3,000 units/L. Gastric bleeding was observed on the third day after admission. The patient’s blood test results and general condition continued to deteriorate. She was transferred to a general hospital. After the transfer, gastric bleeding, hematemesis, and melena were continuously observed. Gastrointestinal bleeding did not improve after a transfusion. Viral tests were all negative. The patient was diagnosed with acute fulminant hepatitis of unknown cause. After intubation due to dyspnea, symptomatic treatment continued in the intensive care unit (ICU). However, the patient’s mental status was drowsy. She died of multiple organfailure due to disseminated intravascular coagulation one day after transfer to the general hospital.
Case 2
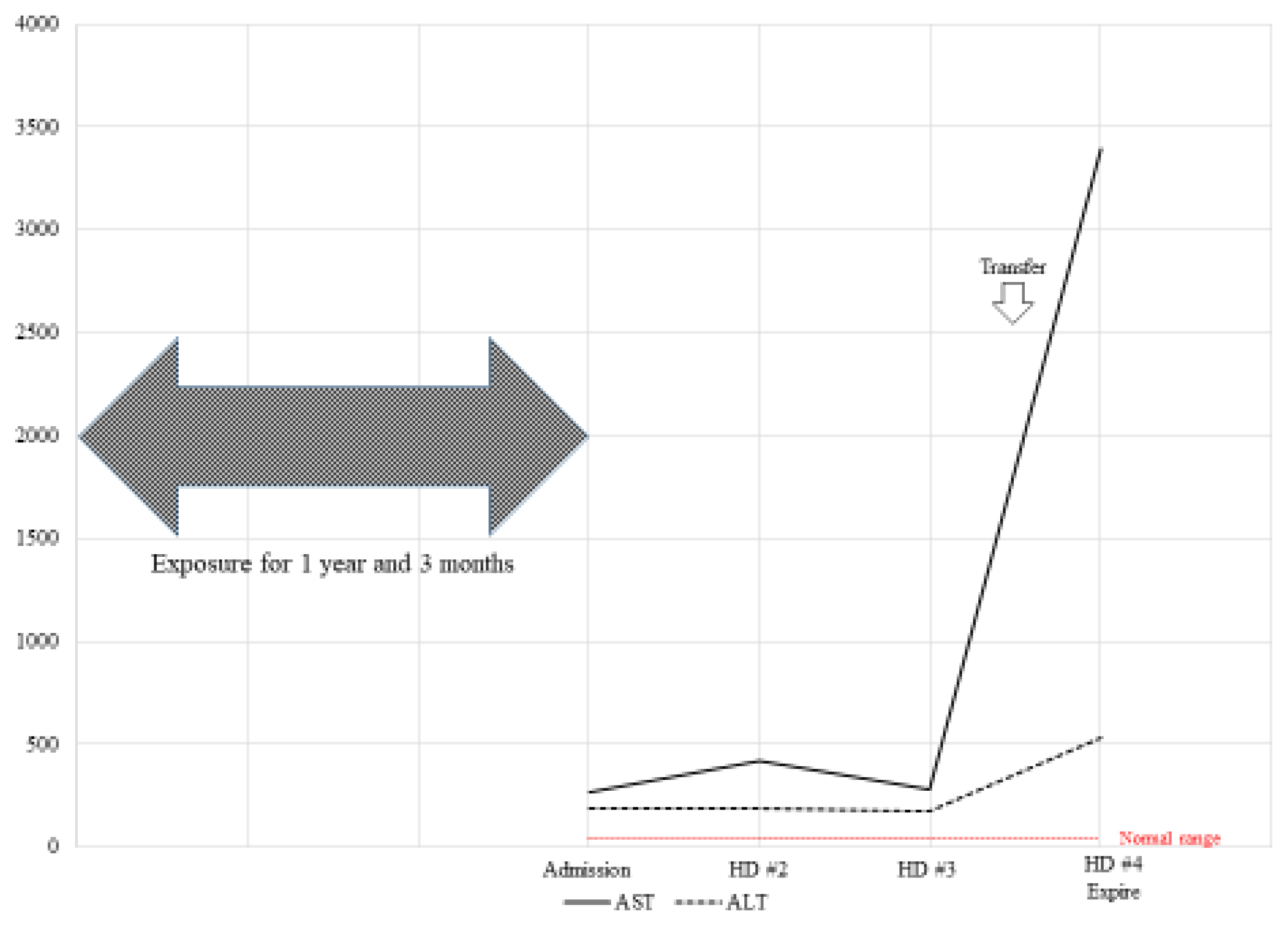
Case 2 was a four-month-old male patient without an underlying disease. His birth weight was 3.06 kg, with normal findings during pregnancy and birth. He was exposed to HD containing 100% PHMG from birth for 24 hours per day. The distance between the humidifier and the patient was two meters.
The patient visited the clinic due to a fever and persistent cough. His AST and ALT levels were confirmed to be 2,061 and 2,245 units/L, respectively (Fig. 2). He was admitted to the hospital for elevated hepatic enzymes. The doctor suspected cytomegalovirus infection and prescribed medications. Viral testing confirmed negative immunoglobulin M and positive immunoglobulin G. The patient was transferred to a general hospital for further medical examination.
The patient had a cough and rhinorrhea with normal body temperature. There was no evidence of infection at evaluation. The patient was discharged upon improvements in symptoms and hepatic enzyme levels. Follow-up testing showed increased hepatic enzyme levels. Hepatomegaly was still observed.
Case 2 patient also experienced respiratory symptoms, such as cough, after exposure to HD, and was treated for pneumonia and rhinitis several times. He was diagnosed with attention deficit hyperactivity disorder (ADHD). He still complains of unrecovered fatigue and lethargy.
Case 3
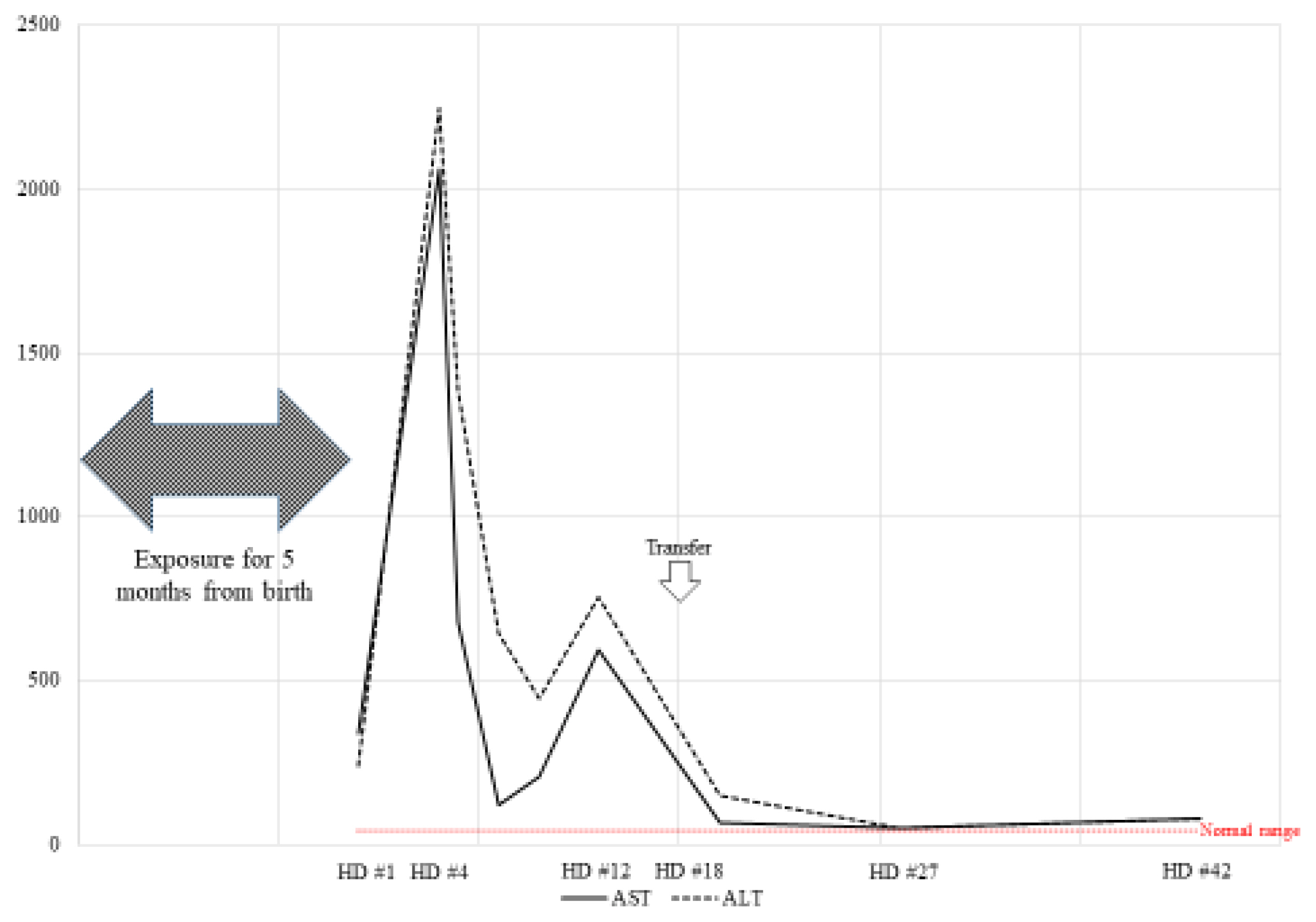
Case 3 was a 41-year-old female exposed to HD since the age of 38 (November 2007 to April 2010). During about 45% of the total exposure period, PHMG was used as an HD ingredient, and during the remaining 55%, CMIT/MIT was used. The patient stated that she was exposed to HD for an average of 11 hours per day in a residential space.
The patient reported upper respiratory symptoms and dyspnea. A pulmonologist began treatment based on the suspicion of asthma. After six months of asthma treatment, prednisolone was tapered due to improvement in symptoms. However, sore throat, cough, sputum, and chest discomfort suddenly recurred. Auscultated rhonchi lung sounds were heard by the pulmonologist. The doctor prescribed prednisolone to improve the symptoms. However, the patient was admitted to the hospital with a worsening cough and elevated hepatic enzymes after one week. Approximately 17 days after hospitalization, her hepatic enzyme level increased from 250 to 4,000 units/L (Fig. 3). Her symptoms persisted, and the fever remained uncontrolled. The patient was transferred to a general hospital.
In the general hospital, the medical staff tried to determine the cause with hepatotoxicity of unknown origin. Granulomatous hepatitis was found on liver biopsy. The liver biopsy results are as follows:
Multifocal fibrin extravasation and necrosis with suspicious granulomatous reaction
Moderate portal inflammation
After the symptoms were controlled, the patient was discharged from the hospital. She voluntarily stopped using HD. Her asthma symptoms persisted. Treatment was continued as her forced expiratory volume in one second (FEV1) decreased to 27%. Prednisolone was continued, and toxic hepatitis has not recurred.
Transmission Electron Microscopy
We observed the mitochondria of two patients (except case 1) by transmission electron microscopy (TEM) and found decreases in the size of the mitochondria. They were distorted with blurred cristae. The ring of the mitochondria was not clear, and the boundary was blurred in both patients (Figs. 4 and 5). Damage to the mitochondrial inner membrane, outer membrane, and cristae indicates that the mitochondrial electron transport chain is disrupted, resulting in impaired ATP production [4,5]. This article is a human case series study that supports prior knowledge that hepatotoxicity can occur through HD inhalation. Alcohol consumption, HBV, and hepatitis C virus (HCV) are known as the major causes of hepatotoxicity. Obesity, diabetes, and some drugs can also induce hepatotoxicity [6]. The highly suspect hepatotoxic drugs include antihypertensive, antirheumatic, analgesic, anticonvulsant, and antimicrobial agents. In particular, minocycline and nitrofurantoin have been reported as major causes of drug-induced hepatotoxicity [7]. Accordingly, the patient’s medical history is important when determining the hepatotoxic cause. We confirmed that there were no underlying factors that could induce hepatitis in the three patients through an interview and medical records. In Oriental culture, herbal medicines have been reported as a major cause of toxic hepatitis. In this regard, all patients denied using herbal medicines.
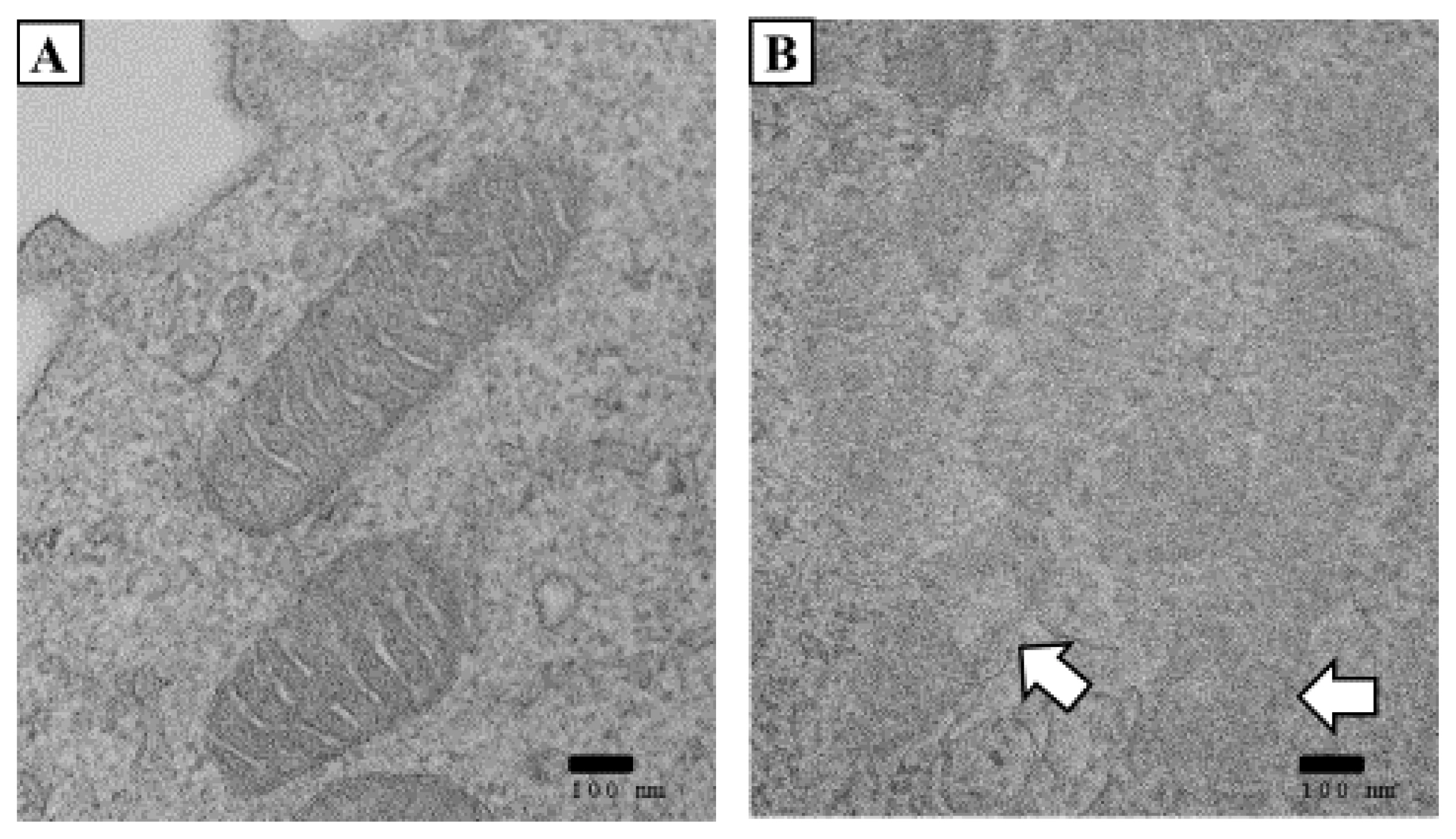
Changes in mitochondrial ultrastructure in the patient in case 2. Typical TEM images of white blood cells (WBCs). (A) Normal mitochondrial structure of the case 3 patient. No normal mitochondria were seen in case 2. (B) Damaged mitochondria’s double membrane structure contained lucent materials. (A) and (B) scale bar, 100 nm. (Date of test: June 5, 2020).
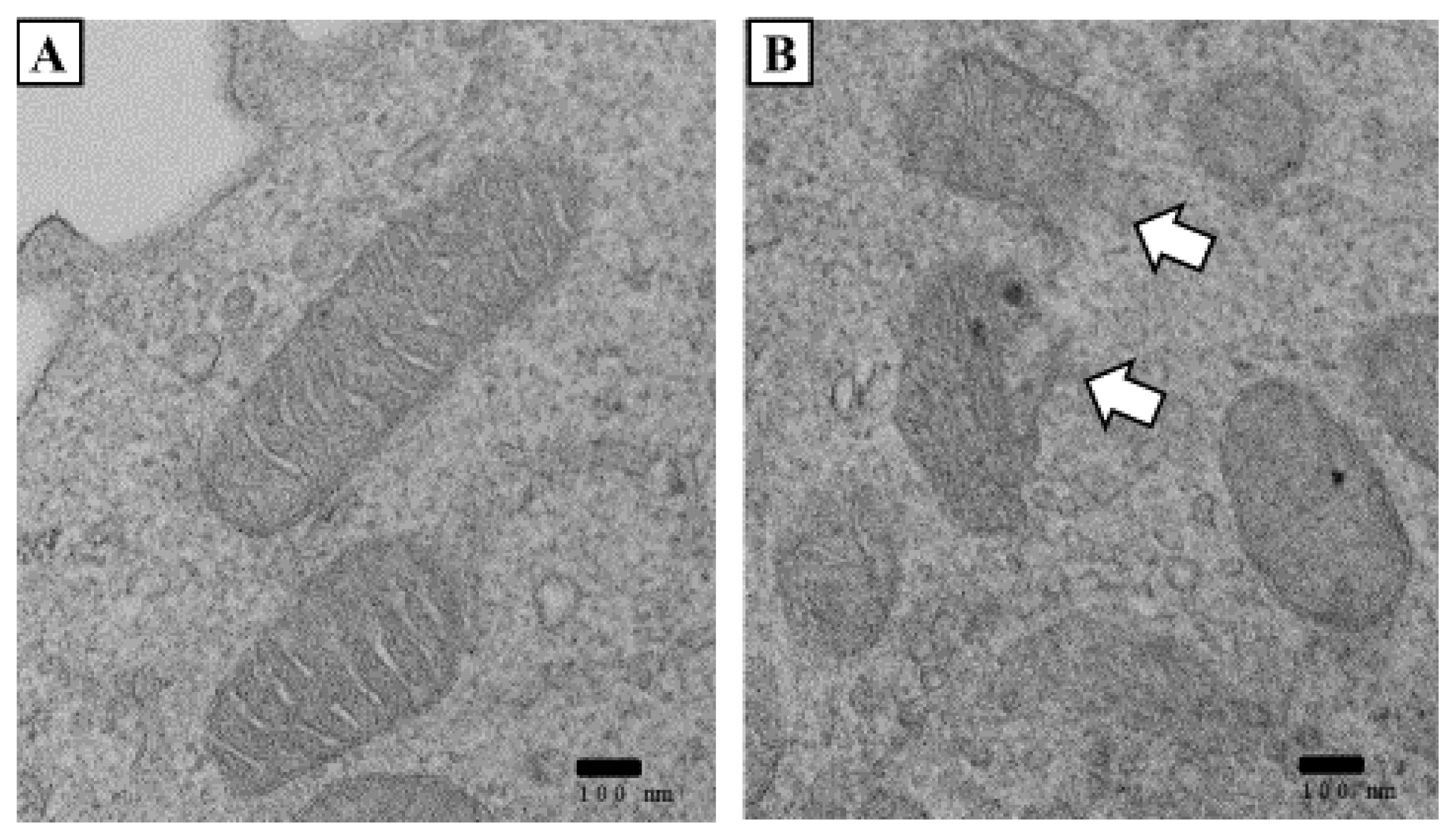
Changes in mitochondrial ultrastructure of the case 3 patient. Typical TEM images of WBCs. (A) Normal mitochondrial structure. (B) Compared to normal mitochondria, the inner and outer layers were fragmented. They contained lucent materials. (A) and (B) scale bar, 100 nm. (Date of test: April 7, 2020).
Discussion
This article is a human case series study that supports prior knowledge that hepatotoxicity can occur through HD inhalation. Alcohol consumption, HBV, and hepatitis C virus (HCV) are known as the major causes of hepatotoxicity. Obesity, diabetes, and some drugs can also induce hepatotoxicity6. The highly suspect hepatotoxic drugs include antihypertensive, antirheumatic, analgesic, anticonvulsant, and antimicrobial agents. In particular, minocycline and nitrofurantoin have been reported as major causes of drug-induced hepatotoxicity [7]. Accordingly, the patient’s medical history is important when determining the hepatotoxic cause. We confirmed that there were no underlying factors that could induce hepatitis in the three patients through an interview and medical records. In Oriental culture, herbal medicines have been reported as a major cause of toxic hepatitis. In this regard, all patients denied using herbal medicines.
Alcoholic hepatitis and viral hepatitis are prevalent in Korea, and this makes it hard to diagnose toxic hepatitis. In Korea, a heavy chemical industry has developed, and toxic hepatitis due to chemical exposure is reported annually by workers. Toxic hepatitis caused by occupational exposure usually occurs two to four weeks after exposure and usually occurs in persons entering the work process for the first time [8]. Unlike toxic hepatitis in occupational exposure, HD exposure occurs for a long time at a low concentration. There is also a difference because exposure occurs in vulnerable groups, such as children and people with respiratory diseases.
In case 3, a drug use history during HD exposure was confirmed, and very careful consideration was given to enrolling this patient in our case series. However, this case was an important example that clearly showed hepatotoxicity caused by exposure to HD by stopping exposure to HD before and after hepatic enzyme elevation. In addition, the liver biopsy results suggested continuous liver damage, and no increases in hepatic enzyme levels were observed again despite continued steroid treatment after the end of exposure to the HD, both of which are important evidence.
Recent animal studies provided evidence that exposure to HD could cause hepatotoxicity, supporting the results of our study [9–12]. After male mice were injected intraperitoneally with PHMG (0.03% and 0.1%) twice a week for five weeks, diffuse fibrotic changes in the liver were observed9. Weight loss and mRNA gene expression changes were also observed, depending on the injection dose. This is strong evidence that PHMG can cause hepatotoxicity, such as fibrosis if it reaches the liver. The possibility that HD could reach extrapulmonary organs has been continuously questioned10. In an animal toxicokinetic study of inhalation exposure using indium-111, after one week, inhaled PHMG isotope accumulated at 74% in rat lungs and 5.2% in rat liver11. This provides evidence that inhaled PHMG can reach extrapulmonary organs. Another animal study also reported that PHMG inhalation showed subacute effects on systemic organs [12]. By summarizing the results of previous studies, it can be inferred that PHMG can reach the liver through inhalation exposure and induce hepatotoxic effects. Findings, such as extravasation necrosis and portal inflammation in a liver biopsy performed during the inpatient treatment of the patient in case 3, support our assertion.
PHMG can induce cellular toxicity. PHMG remains in systemic organs for a long period. It can also induce chronic cellular inflammation. PHMG promotes the production of reactive oxygen species and consequently changes the expression of DNA methylation or damage markers, such as ATM and H2AX phosphorylation [13,14]. Among the intracellular organs, mitochondria are extremely sensitive to oxidative damage caused by the absence of protective histone, and an incomplete repair mechanism [15].
We observed the mitochondria of two patients (except for case 1) by TEM and found that the mitochondria were decreased in size. They were distorted with blurred cristae. The ring of the mitochondria was not clear, and the boundary was blurred in both patients (Figs. 4 and 5). Damage to the mitochondrial inner membrane, outer membrane, and cristae indicates that the mitochondrial electron transport chain is disrupted, resulting in impaired ATP production [4,5].
In this report, we present the overlooked toxic effects of HD inhalation exposure. This case series included patients who were diagnosed with toxic hepatitis, excluding other major causes of the condition. Existing concepts have only suspected lung disease depending on the exposure route. However, our study and several previous studies provide clear evidence for extrapulmonary toxicity. In future studies, damage to extrapulmonary organs due to “humidifier disinfectant syndrome” should be investigated [16]. This report can be used as a foundation for future research. The health effects of HD need to be more extensively clarified in the future.
Acknowledgement
The authors wish to thank the National Institute of Environmental Research for their assistance with this study.
Notes
The author declares no conflict of interest.
CRediT author statement
HDK: Data curation, Formal analysis, Writing-original draft, Writing-review and editing; HCK: Formal analysis, Writing-review and editing; JHL: Conceptualization, Writing-review and editing.