Occurrence of veterinary antibiotics in poultry manure from two farms in Ibadan, Nigeria: Ecotoxicological implications in manure-amended soil
Article information
Abstract
Veterinary antibiotics are commonly used in poultry farming for preventing diseases and promoting growth. As a result of their incomplete metabolism in poultry birds, veterinary antibiotics are usually excreted and are frequently detected in poultry manures. Veterinary antibiotics in poultry manure applied onto soil may pose serious ecological effect to the terrestrial and aquatic environment. In the present work, the occurrence of three veterinary antibiotics (sulfamethoxazole, sulfadimidine and trimethoprim), categorized as veterinary antimicrobial agents of critical importance, was investigated in poultry manure from two poultry farms in Nigeria. The potential ecotoxicological risk of target veterinary antibiotics in poultry manure-amended soil was also assessed. A modified quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction was adopted for the extraction of target veterinary antibiotics and instrumental analysis was achieved by high performance liquid chromatography. Sulfamethoxazole, sulfadimidine and trimethoprim were quantified in poultry manures from the poultry farms up to 12.7 μg g−1, 16.1 μg g−1 and 33.8 μg g−1, respectively. Sulfamethoxazole and trimethoprim in poultry manure-amended soil presented low risk to Eisenia fetida (earthworm). The ecological effect of sulfamethoxazole for the root length of rice was high in Farm B and medium in Farm A. Sulfamethoxazole presented high risk to aquatic organisms while sulfadimidine and trimethoprim posed medium risk and low risk, respectively to aquatic organisms. The results indicated that residual veterinary antibiotics in poultry manures could have adverse effects on crops after application to agricultural soil. There is a need for effective enlightenment programs for poultry farmers in Nigeria to bring about awareness on the environmental and toxicological impact of the excessive and uncontrolled use of veterinary antibiotics in poultry farming and the adverse ecological implications of poultry manure application on farmlands.
Introduction
Veterinary antibiotics are intensively used in poultry farming to prevent and treat microbial infections as well as to increase the efficiency of feeds in promoting the growth of poultry birds [1,2]. These antibiotics are not completely metabolized in the body tissues of poultry birds, they get deposited in poultry meat as parent compounds and are ultimately excreted via poultry droppings into the environment [3,4]. These poultry droppings are commonly applied as manures on farmlands [5]. Although poultry manure is a rich source of essential nutrients such as nitrogen and phosphorus to soil, other potential environmental toxic substances such as antibiotics, pathogens, hormones, and heavy metals are often present in the poultry droppings [3].
Special attention has been drawn around the globe to the potential harmful effect of antibiotic residues on the living environment [3,6]. The use of veterinary antibiotics has created public and environmental health concerns [1]. The release or accumulation of veterinary antibiotics through the application of manure on farmlands may increase the risk of spreading antibiotic-resistant genes in the environment [2,7]. The uptake of veterinary antibiotics by plants grown in manure-amended soil also raises potential human health concerns [2,8]. After the application of animal manure onto soil, veterinary antibiotics have the potential to still remain in the soil due to ineffective degradation and thus may pose risks to the terrestrial environment [9,10]. Residual antibiotics in poultry manure could also have adverse effects on crops after application to agricultural soil [11]. For example, sulfamethazine antibiotic in poultry manure can change carbon and nitrogen mineralization in soil [2].
Sulfonamides are a class of antibiotic substances widely used in treating bacterial diseases in animal husbandry [10]. Trimethoprim is also commonly used either individually or in combination with a sulfonamide in treating bacterial infections [12]. In the European Union, sulfonamides and trimethoprim were found to account for 11% and 1.6%, respectively of the total antibiotics consumption for food producing animals in 2012 [4,13]. Trimethoprim often acts as a bactericidal agent in the presence of sulfonamides (such as sulfadimidine and sulfamethoxazole) against Gram-positive and many Gram-negative bacteria through the inhibition of nucleic acid synthesis [14]. Despite the importance of veterinary antibiotics in poultry production, risk assessment of these antibiotics towards soil microbial communities and other soil dwelling organisms due to application of poultry manure onto soil is still lacking [4]. There are currently scarce studies on the concentration of antibiotics in the poultry litter and the residual antibiotic concentration in poultry manure used for agricultural purposes [14].
In Nigeria, veterinary antibiotics are routinely used in livestock and poultry production especially as additives to feed and water [15], and thus, the poultry production environment in Nigeria represents an important reservoir of antibiotics and antibiotic resistance genes that may spread to human populations via manure application on production farms [15]. Land application of poultry manure is a common practice in Nigeria because of its value in supplying nutrients to crops as well as being an inexpensive means of poultry waste disposal. This practice of land application of poultry manure poses potential risk to soil dwelling organisms, crops and possibly aquatic organisms. Hence, the occurrence of these antibiotics in poultry manure derived from poultry farms in Nigeria is worth investigating. To the best of our knowledge, no earlier study exists till date on the occurrence of veterinary antibiotics in poultry manure from poultry farms in Nigeria and ecotoxicological effect of the application of poultry manure on soil for agricultural purposes in Nigeria. Antibiotics were only recently determined in sewage sludge, hospital wastewater and dumpsite leachates in Nigeria [12,16,17].
The objective of this study was, therefore, to investigate the occurrence of three veterinary antibiotics sulfamethoxazole, sulfadimidine and trimethoprim which have been categorized as veterinary critically important antimicrobials [18] in poultry manures from two poultry farms in Ibadan, Nigeria. Potential ecotoxicological effect of these antibiotics in poultry manure-amended soil to terrestrial organisms (crops and soil dwelling organisms) and aquatic organisms due to land application of poultry manures from two farms in Nigeria was also investigated. A modified quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction protocol was employed for the extraction of target antibiotics from the poultry manures while instrumental analysis was carried out by high performance liquid chromatography. To the best of our knowledge, first report is presented on the presence of veterinary antibiotics sulfamethoxazole, sulfadimidine and trimethoprim in poultry manure from poultry farms in Ibadan, Nigeria and their ecotoxicological risk assessment in poultry manure-amended soil.
Materials and Methods
Chemicals and reagents
Analytical standards of target antibiotics (sulfamethoxazole, sulfadimidine and trimethoprim) were of high purity (≥98%) and were purchased from Sigma Aldrich, (Steinheim, Germany). HPLC grade acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Citric acid monohydrate (C6H8O7·H2O), disodium hydrogen phosphate (Na2HPO4) and ethylenediaminetetraacetic acid disodium dihydrate (Na2EDTA·2H2O) salts of analytical grade were purchased from Sigma Aldrich (Steinheim, Germany). Sodium chloride, 99.5% for analysis and anhydrous magnesium sulfate (MgSO4) were obtained from ACROS OrganicsTM. Dispersive-SPE sorbent of primary and secondary amine (PSA) was purchased from Supelco (Bellefonte, USA) while octadecyl silica C18 (EC) sorbent was bought from International Sorbent Technology (IST) UK. Individual standard solutions of target antibiotics (sulfamethoxazole, sulfadimidine and trimethoprim) with a concentration of 1000 mg L−1 were prepared in acetonitrile. Stock solution (100 mg L−1) containing all three antibiotics was prepared from the individual standard solutions. Working standard solutions were prepared by serial dilution of the stock solution. McIlvaine buffer (pH 4) was prepared by dissolving 7.5 g disodium hydrogen phosphate dihydrate and 6.5 g citric acid monohydrate in 500 mL of water. 0.1 M Na2EDTA-McIlvaine buffer was prepared by dissolving 18.61 g Na2EDTA in 500 mL of McIlvaine buffer.
Samples collection and sample pre-treatment
Poultry litters were collected from two poultry farms (Farm A and Farm B) located at Egbeda-Awaye, Egbeda Local Government Area, Ibadan, Oyo State, South-Western Nigeria: Farm A (Lat.: 7.4481436, Long.: 4.0662889) and Farm B (Lat.: 7.44763, Long.: 4.0669977). Questionnaires were initially administered to interview the farmers if antibiotics were being used for their agricultural practices, types of antibiotics used, the dosage administered, the frequency of antibiotics administration and whether the poultry manures were used for crop production. Many poultry farms were visited for the interview in Ibadan metropolis. Farms A and B were selected for their practice of using antibiotics in treating their poultry birds and their use of waste excreta as poultry manure for growing vegetables and other crop produce. A map of the study location is shown in Figure 1.
Poultry manure samples were collected from each of the farms in the months of July and August 2019. From each sampling site and during each sampling, poultry litter samples from different locations along the pile were scooped into a polyethylene bag and thoroughly mixed together. To avoid degradation of target antibiotics, poultry litter samples were kept in a cooler containing ice and immediately transported under cooled conditions to the laboratory. The samples were air dried. The dried samples were ground using a mortar and pestle, wrapped in aluminum foil and stored in a refrigerator prior to extraction and instrumental analysis. Samples for the experiments on method performance were prepared by mixing together the samples from both sampling sites.
QuEChERS extraction
Extraction of target antibiotics was carried out employing the QuEChERS extraction procedure by Guo et al. [19] with slight modifications. Briefly, 1 g of sample was weighed into a 50 mL centrifuge tube. Samples for the experiments on method performance were spiked with appropriate volume of working standard solution while 100 μL of water was added to the samples for real analysis. The sample was stirred vigorously and then placed in darkness overnight. A total of 20 mL extracting solvent comprising methanol/acetonitrile/0.1 M Na2EDTA-McIlvaine buffer in a percentage fraction of 12.5/37.5/50 respectively was added to the sample in the centrifuge tube. The sample was vortex-mixed for 2 minutes and centrifuged for 25 minutes at 3500 rpm. After the centrifugation was complete, the supernatant was decanted into another 50 mL centrifuge tube. The extraction step was repeated. 20 mL of a mixture of methanol/acetonitrile/0.1 M Na2EDTA-McIlvaine buffer (12.5/37.5/50%) was added and the tube was vortex-mixed and centrifuged. The organic layer was then decanted once more into the centrifuge tube containing the previously decanted supernatant.
d-SPE clean-up
The supernatant in the centrifuge tube was then subjected to liquid-liquid partition with 1 g of NaCl and 4 g of MgSO4. The tube was capped and shaken by hand for 1 min to prevent formation of crystalline agglomerates during MgSO4 hydration. The tube was centrifuged for 10 minutes at 2500 rpm. The organic layer was transferred into another centrifuge tube containing clean-up sorbent (40 mg PSA and 20 mg C18), vortex-mixed for 1 minute and centrifuged for 10 minutes at 2500 rpm. The sample was gently evaporated, reconstituted with 1 mL mixture of acetonitrile and 0.1% formic acid (20:80, v/v) and transferred into a glass vial for HPLC analysis.
High performance liquid chromatographic analysis
Target antibiotics were analyzed using high performance liquid chromatography (HPLC), Agilent series 1100 equipped with ultraviolet (UV) and fluorescence detectors. The analytes were separated on a Zorbax Eclipse XDB-C18 (150 mm×4.6 mm, 5 μm) column. UV detection of the three antibiotics was achieved at 254 nm wavelength. Isocratic elution was used with a mobile phase containing an aqueous solution of 0.05 M potassium dihydrogen phosphate buffer (adjusted to pH 3.0 with 0.1% formic acid) and acetonitrile (40:60, v/v). Flow rate of mobile phase was 1.0 mL/min and the injection volume was 10 μL.
Quality assurance
Recovery and precision were determined by spiking manure matrix in replicates at 5 μg g−1 fortification level. The recovery of each analyte was estimated by using the following formula (Equation 1):
Precision of the method was appraised in terms of repeatability which was calculated as the percentage relative standard deviation (%RSD) of results obtained for each analyte after the replicate analysis. The limit of detection (LOD) and limit of quantification (LOQ) of the method were determined by spiking manure samples prior to extraction at concentration of 5 μg g−1. The LOD and LOQ for each analyte were calculated by multiplying standard deviation of the concentrations of replicate samples by a factor of 3.3 and 10 respectively divided by slope of the calibration curve. Linearity was checked using external calibration curves at four calibration points ranging from 12.5μg mL−1 to100 μg mL−1. Calibration curves were constructed by plotting the peak area of each analyte against the corresponding analyte concentration.
Ecotoxicological risk assessment in poultry manure-amended soil based on terrestrial toxicity data
Ecotoxicological risk of target antibiotics in poultry manure-amended soil was assessed. Toxicity data (EC50/LC50/NOEC) for terrestrial organisms were sourced from literature, and this includes toxicity data for earthworms and crop plants (root elongation and seedling heights). The terrestrial toxicity data are presented in Table 1.
Predicted-no-effect-concentration of target antibiotic based on terrestrial toxicity data (PNECsoil-terrestrial) was calculated by dividing toxicity data (EC50/LC50/NOEC) by a suitable assessment factor as shown in Table 1, using (Equation 2) with the calculated values provided in Table 1.
Predicted environmental concentrations of target antibiotics in poultry manure-amended soil (PECsoil) were calculated by using the following equation (Equation 3) according to Ghirardini et al. [20] and the European Technical Guidance Document on risk assessment [21]:
where Co(soil) represents the background concentration of antibiotic in the soil before application of poultry manure (was taken to be zero), MEC(manure) stands for the measured environmental concentration in poultry manure (μg kg−1), APP (manure) is the typical application rate of dry manure onto soil which is 0.5 kg m−2, DEPTH(soil) is the mixing depth of 0.2 m generally used for agricultural soil, RHO(soil) is the bulk density of wet soil (1700 kg m−3). Risk quotient of target antibiotics in poultry manure-amended soil for terrestrial organisms (RQsoil-terrestrial) was calculated using Equation 4.
Risk was categorized according to Ghirardini et al. [20]: high risk if RQ≥1, medium risk if 0.1<RQ<1, and low risk if RQ≤0.1.
Ecotoxicological risk assessment in poultry manure-amended soil based on aquatic toxicity data
Terrestrial toxicity data of target antibiotics are scarce in literature. Therefore, aquatic toxicity data were also used to estimate PNECsoil-aquatic through the equilibrium partition approach (Equation 5) in the European guidance on information requirements and chemical safety assessment (ECHA 2008, Chapter R.10) [25]. Aquatic toxicity data were collected for fish, daphnia and algae and are presented in Table 2. The lowest toxicity value out of the three values for each antibiotic was used to estimate the worst case scenario. PNECwater was calculated by dividing the lowest aquatic toxicity data by assessment factor of 1000.
where Ksoil.water is soil–water partition coefficient, RHOsoil stands for the bulk density of wet soil (1700 Kg m−3). Ksoil.water was calculated using Equation 6 according to the European Chemical Agency [26].
where Fairsoil stands for the volume fraction air in soil (0.2), Kair-water represents the air-water partition coefficient (zero for non-volatile substances), Fwatersoil (0.2) and Fsolidsoil (0.6) represent the volume fraction water in soil and the fraction solid in soil, respectively. Foc(soil) is the weight fraction organic carbon in soil (0.02), Koc is the organic water partition coefficient in Table 2 while RHOsolid is the density of solid phase (2500 kg m−3). If all the default values in bracket are substituted into Equation 6, Ksoil.water can be calculated. Substituting the calculated value of Ksoil.water into Equation 5, the equilibrium partition equation is simplified into Equation 7:
where Koc stands for the organic carbon partition coefficient of antibiotic (as L kg−1). Table 2 shows the calculated PNECsoil–aquatic values for target antibiotics.
Risk quotient (RQ) of target antibiotics in poultry manure-amended soil based on aquatic toxicity data (RQsoil-aquatic) was calculated by using Equation 8:
Risk was categorized according to Ghirardini et al. [20]: high risk if RQ≥1, medium risk if 0.1<RQ< 1, and low risk if RQ≤0.1.
Results and Discussion
Quality assurance parameters
The analytical quality parameters for the determination of target antibiotics in poultry manure are provided in Table 3. Recoveries of target antibiotics at 5 μg g−1 spike level ranged from 77.4% to 136.5%. Hou et al. [39] also reported recoveries range between 63.1% and 79.6%. Precision in terms of percentage relative standard deviation (%RSD) was ≤ 24%. The LODs for the determination of sulfamethoxazole, sulfadimidine and trimethoprim were 0.46 μg g−1, 0.04 μg g−1 and 0.18 μg g−1, respectively while the corresponding LOQs for sulfamethoxazole, sulfadimidine and trimethoprim were 1.40 μg g−1, 0.13 μg g−1and 0.55 μg g−1, respectively. Similar LOD and LOQ values were reported for trimethoprim by Teglia and co-workers [40]. Good linearity of R2 greater than 0.994 was achieved in the calibration curves for all compounds.
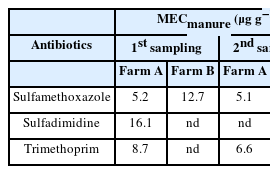
Occurrence of target antibiotics in poultry manure and manure-amended soil
The measured environmental concentrations of the antibiotics in poultry manure from the two farms and predicted environmental concentrations in manure-amended soil are presented in Table 4. Sulfamethoxazole was measured up to 5.2 μg g−1 and 12.7 μg g−1 in poultry manure from the two farms in first and second sampling. Similar levels of sulfamethoxazole were also quantified in poultry manure in other studies, up to 7.11 μg g−1 by Li et al. [41] and 3.76 μg g−1 by Karci and Balcioglu [42]. Sulfadimidine was not detected in poultry manure from both farms in the second sampling and in Farm B in the first sampling. High concentration of 16.1 μg g−1 was, however, measured for sulfadimidine in poultry manure from Farm A in the first sampling. High concentrations of 8.62 μg g−1 and 5.650 μg g−1 were also measured for sulfadimidine in chicken manure by Ji et al. [43] and Zhou et al. [11], respectively but much lower concentrations of sulfadimidine in chicken manures have been reported by other researchers [39,44–47]. Sulfadimidine was among the veterinary antibiotics investigated in poultry manure by Topi and Spahiu [48] but was not detected in the samples. Trimethoprim was quantified in this present study up to 8.7 μg g−1 in Farm A and 33.8 μg g−1 in Farm B. Lower amounts of trimethoprim were quantified in poultry litters in some previously published studies [39,40], and was not detected by Patyra et al. [49]. The predicted environmental concentrations (PECsoil) of target antibiotics in poultry manure-amended soil were generally low and are presented in Table 4. PECsoil values for sulfamethoxazole ranged between 0.0075 μg g−1 and 0.0187 μg g−1, while the PECsoil value was calculated up to 0.0237 μg g−1 and 0.0497 μg g−1 for sulfadimidine and trimethoprim, respectively.
Ecotoxicological risk in poultry manure-amended soil based on terrestrial toxicity data
The values of risk quotients of target antibiotics to terrestrial organisms (RQsoil-terrestrial) are presented in Table 5. Sulfamethoxazole in poultry-amended soil in Farm A and Farm B presented low risk to earthworms and medium ecological effects to the seedling height of rice and cucumber. The risk of sulfamethoxazole for the root length of rice was high in Farm B and medium in Farm A while low ecological effect was observed for the root length of cucumber. Sulfadimidine posed medium ecological effect to the seedling height and root length of rice in Farm A and low ecological effect to the seedling height and root length of cucumber in Farm A. In a study by Zhou et al. [11], sulfadimidine was found to pose high risk to rice roots and medium risk to rice seeds and cucumber while sulfamethoxazole presented medium risk to rice roots but low risk to rice seeds and cucumber [11]. Trimethoprim presented low ecological risk to earthworm in poultry manure-amended soils of Farm A and Farm B. Trimethoprim also posed low ecological effect to the seedling height and root length of both rice and cucumber in Farm A, but medium ecological effect to the seedling height and root length of both rice and cucumber in Farm B. The results indicated that residual antibiotics in poultry manure could have adverse effects on crops after their application to agricultural soil.
Ecotoxicological risk in poultry manure-amended soil based on aquatic toxicity data
The logarithms (to base 10) of risk quotient values (RQsoil-aquatic) of the target antibiotics in poultry manure-amended soil based on aquatic toxicity data are shown in Figure 2. RQsoil-aquatic values for sulfamethoxazole were greater than 1 in all poultry manure-amended soils. Our results showed that sulfamethoxazole presented high risk to aquatic organisms while sulfadimidine and trimethoprim posed medium risk and low risk, respectively to aquatic organisms. It should be made clear that toxicity data for soil dwelling organisms cannot be replaced by toxicity data for aquatic organisms because the effects on aquatic organisms can only be considered as effects on soil organisms that are exposed exclusively to the soil pore water of the soil [25]. A RQsoil-aquatic value of greater than one (>1) calculated from equilibrium partition approach implies tests with soil dwelling organisms should be considered an essential requirement for a refined hazard assessment. In this study, only sulfamethoxazole had RQsoil-aquatic greater than one and this antibiotic should be considered for tests with soil dwelling organisms. We evaluated RQsoil-terrestrial for sulfamethoxazole to Eisenia fetida the seedling height and root length of rice and cucumber. The RQsoil-terrestrial values were less than 0.005 for Eisenia fetida as presented in Table 5, indicating that the presence of this antibiotic in the poultry manure-amended soil posed no significant risk to earthworm. Low ecological effect of sulfamethoxazole to the seedling height and root length of cucumber was also observed. However, medium to high ecological effect of this antibiotic to the seedling height and root length of rice was likely.
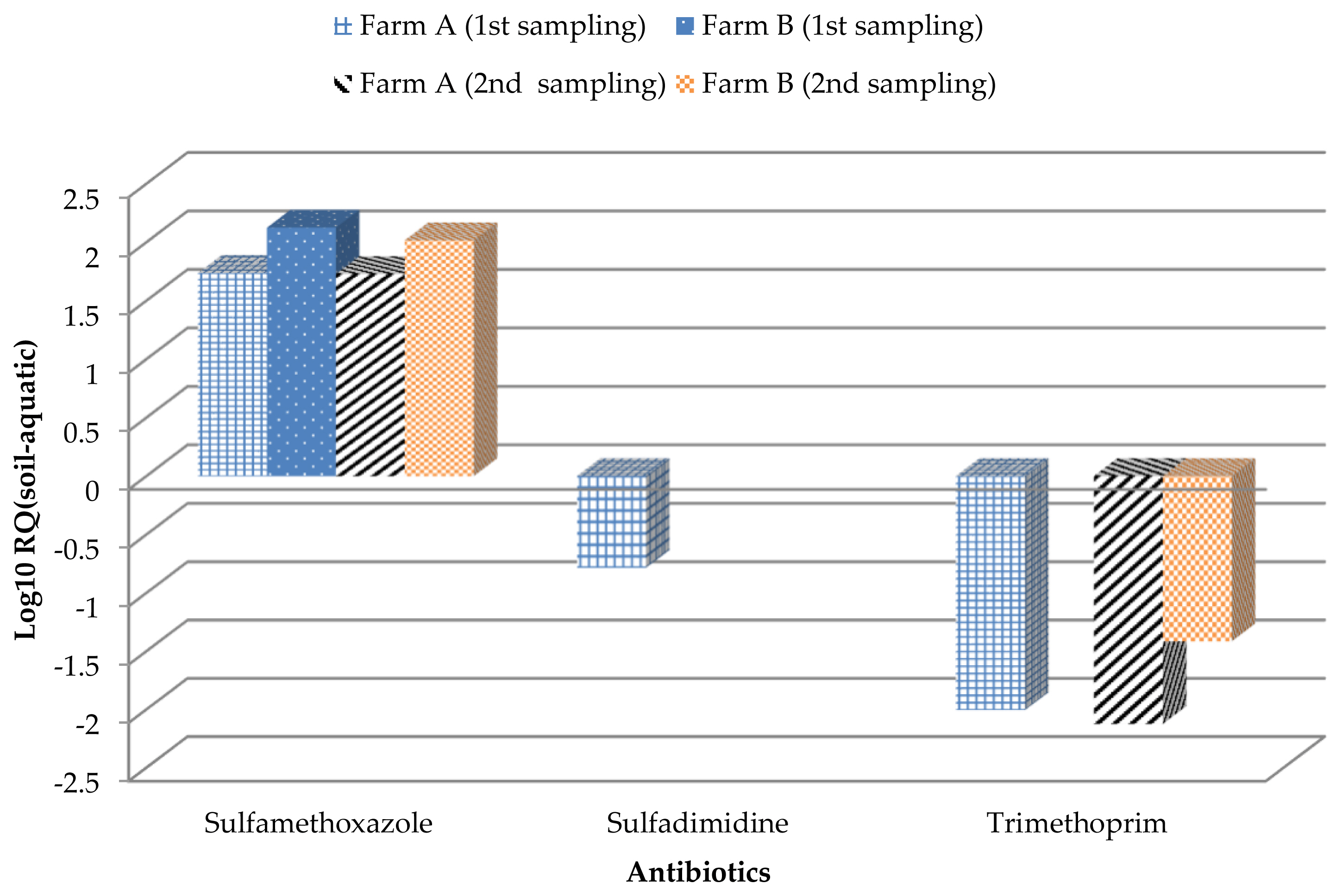
RQsoil-aquatic of target veterinary antibiotics in poultry manure-amended soil based on aquatic toxicity data.
Considering the benefits derived from the application of poultry manure on soil for agricultural practices but also the possible consequential threats to soil, terrestrial and aquatic environment, poultry manure should not be applied in excess and uncontrolled manner. Future studies on the occurrence and risk assessment should also include other relevant veterinary antibiotics commonly used in poultry production to have a more comprehensive overview of the effect veterinary antibiotics could pose to soil and terrestrial environment.
Conclusions
The concentration profile of three antibiotics (sulfamethoxazole, sulfadimidine and trimethoprim) was determined in poultry manure from two farms in Ibadan, Nigeria. Potential ecotoxicological risk of target antibiotics in poultry manure-amended soil, based on terrestrial toxicity data and aquatic toxicity data, was also investigated. The analytical quality parameters were satisfactory for the determination of target antibiotics in poultry manure. High levels of these antibiotics were quantified in poultry manures from the farms, up to 12.7 μg g−1, 16.1 μg g−1 and 33.8 μg g−1 for sulfamethoxazole, sulfadimidine and trimethoprim, respectively. Sulfamethoxazole and trimethoprim posed low risk to earthworms. Only sulfamethoxazole presented high risk to the aquatic organisms but may cause low ecotoxicological effect to crop plants except for the root length and seedling height of rice. We hereby recommend effective enlightenment programs for poultry farmers in Nigeria to bring about awareness on the environmental and toxicological impact of the excessive use of veterinary antibiotics in poultry farming and the adverse ecological implications of poultry manure application on farmlands.
Acknowledgement
Personnel of the investigated poultry farms are appreciated for their cooperation and assistance in the collection of poultry manure samples.
Notes
Conflict of interest
The authors declare that there is no conflict of interest
CRediT author statement
AA: Conceptualization, Methodology, Supervision, Data curation, Writing-Original draft preparation, Writing-Review & Editing; DSO: Investigation, Writing-Original draft preparation; OAB: Investigation, Writing-Original draft preparation.