Comparison of mice’ sperm parameters exposed to some hazardous physical agents
Article information
Abstract
The present study was aimed to compare the effects of exposure to noise, vibration, lighting, and microwave on male mice’ sperm parameters. The mice were randomly assigned to five groups of eight, which comprised of the unexposed group and exposure groups including the lighting (1000 lux), noise (100 dB(A)), vibration (acceleration of 1.2 m/s2) and microwave (power density of 5 watts). The exposure groups were subjected to the four agents for 8 hours a day, 5 days a week during a 2-week period. Semen analysis were done according to World Health Organization guidelines. The highest significant mean difference in sperm count (−1.35×106/mL) had being observed between the microwave group and the control one (P=0.001). The highest difference in immotile percent (25.88 %) had being observed between the noise group and the control one (P=0.001). The highest difference in normal morphology (−27.06 %) observed between the lighting exposure group and the control group (P=0.001). The four agents can cause changes in different sperm parameters, however for definite conclusion; more laboratory and field studies are required. In total, exposure to microwave has had the greatest effect on sperm count and exposure to light has had the greatest effect on normal morphology and non-progressive motility. Moreover, exposure to noise has had the greatest effect on progressive motility and immotile percent, respectively.
Introduction
The advancement of technology in all areas and the process of industrialization has led to the widespread use of different devices, tools and machinery in various industries [1]. This phenomenon has caused humans to be increasingly exposed to varying degrees of hazardous agents both in the workplace and in everyday life. On the other hand, in the preceding decades, studies on the effects of occupational exposure on the reproductive system have been expanded greatly [2], especially since any damage to the reproductive system can lead to permanent or temporary infertility, genetic mutations or hereditary cancer [3]. Research has shown that the quality of sperm has deteriorate over the past fifty years and this has raised questions regarding the negative effects of hazardous physical and chemical agents on it [4].
Common harmful physical agents at workplaces and environments include microwaves, lighting, noise and vibration. Noise pollution is defined as exposure to unwanted or unpleasant sound [5] and is dependent on various factors like noise exposure duration and frequency characteristics. Exposure to noise levels exceeding occupational exposure limits leads to reduced efficiency among workers and the enterprise as a whole [6]. Noise exposure can cause cardiovascular, gastrointestinal, behavioral, psychological and sleep related disorders. It can also cause reduced hearing, visual impairment, disruption of the vestibular system and can affect sperm parameters [7,8,9]. The results of a study conducted by Swami et al. regarding the effects of noise exposure on steroidogenic hormones in men showed that exposure to noise at 100 dB(A) can cause a meaningful reduction in serum testosterone levels [10].
Vibration is another hazardous occupational agent in industrialized and developing countries that can cause discomfort and dissatisfaction among workers [11]. Occupational exposure to Whole Body Vibration (WBV) can be found among workers engaged in vibrating platforms and stone cutting machines as well as drivers [12]. The body response to vibration depends on many variables such as vibration frequency, vibration amplitude, exposure duration and body posture [13]. The effects of vibration on sperm parameters have been proven in previous studies. The results of a study by Penkov et al. regarding the effects of WBV exposure on sperm morphology showed an increase in Oligospermia and Azoospermia, a reduction of ejaculate volume, reduced motile spermatozoa and an increase in the rate of sperm deformation in the exposure group [14].
Lighting can also be a harmful physical occupational factor, though is itself an essential element of occupational safety as it helps in the detection of sizes, shapes or colors, and can increase the accuracy of the workers and help prevent visual errors and occupational accidents [15]. An adult uses his eyes for around 16 hours each day, so the level of lighting must be suited to the precision of work being performed [16]. Advancements in technology and the need for extended or 24-hour work shifts means that workers are subject to prolonged periods of exposure to high levels of lighting, especially in occupations requiring precision such as watch making, cartography and electronics [17]. Brandt et al. conducted a study on the effects of artificial lighting (300 to 350 lux) on the semen quality of adult boars. Their findings showed reduced semen volume and reduced number of motile sperm in the exposure group [18]. Alternative studies however, suggest that exposure to low intensities of visible spectrum and infra-red light can actually increase sperm motility [19].
Technological advancement and the ever-increasing usage of tools and devices has made exposure to electromagnetic fields at home, work, hospitals or industries, inevitable. This has made researchers and the public increasingly concerned regarding the potential biological effects of exposure to these fields [20]. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) insists on the seriousness of the problem regarding electromagnetic radiation and its negative effects on human health and consider it to be a prevalent environmental risk [21]. Therefore, controlling the exposure to electromagnetic fields is as important as the controlling of other hazardous occupational and environmental agents from a health and safety perspective. The daily use of electronic devices has caused people to become increasingly exposed to electromagnetic waves [22]. The electromagnetic spectrum covers a wide frequency range among which, microwave radiation falls between 300 MHz to 300 GHz [23]. A study by Dasdag et al. showed that whole body exposure to microwave radiation had no meaningful effect on sperm count [24]. A study by Mailankot et al. however showed that exposure to microwave radiation can reduce the quality of the ejaculate [25].
Considering the fact that workers are exposed to various occupational risk factors such as noise, vibration, lighting or microwave, and since the results of other studies investigating the effects of these factors on semen indices are conflicting; the present study was designed with the aim of comparing the effects of exposure to these hazardous agents on male mice’ sperm parameters.
Materials and Methods
This case-control study was conducted on 40 male adult NMRI (Naval Medical Research Institute) mice with a weight of 30±2 g and an age of 50 days old. The mice were provided from Neuroscience Research Center, Shahid Beheshti University of Medical Sciences. The mice were randomly assigned to five groups of eight, which comprised of the unexposed group (control group) and the lighting, noise, vibration and microwave exposure groups. The exposure groups were subjected to the four harmful occupational factors for 8 hours a day, 5 days a week for a total of 80 hours of exposure during a 2-week period. This exposure duration was chosen because the regeneration cycle for the Epithelium cells inside the seminiferous tubules of male mice is 8.6 days. This also better approximates the exposure conditions of workers in various industries.
Storage and test conditions
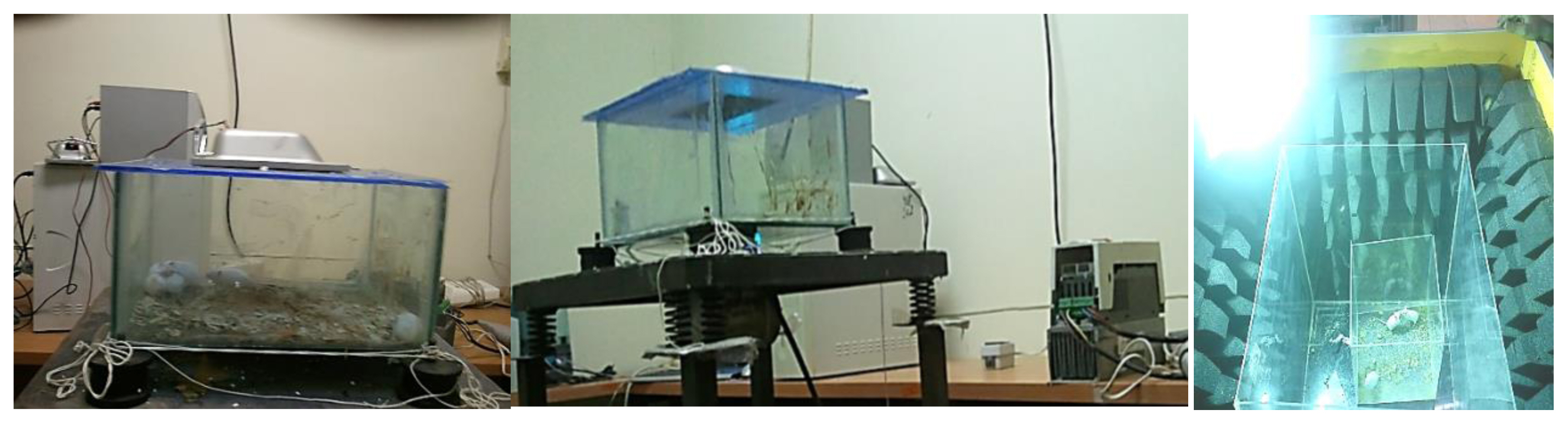
The test mice were initially kept at the animal house under standard conditions (22±2°C, 40–60% humidity, <35 dB(A) background noise, 12-hour light/dark cycle, 100 lux lighting intensity during light cycle, free access to plate and water) [26]. All protocols were in accordance to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and approved by student research committee, Shahid Beheshti University of medical science. The storage space where the mice were kept was a box made of double-pane glass (for internal visibility) with a dimension of 100×90×60 cm and enough space for eight lab mice in each group. Holes were made in each box for observation, measurement (12 times per hour) and ventilation. The storage boxes were designed in a way as to be able to dynamically control the flow of air and to be able to maintain steady temperature and humidity (Figure 1). The mice were killed by the guillotine on the last day of exposure.
Noise exposure
The noise exposure group was subjected to compound noise with a frequency of 500 to 8000 Hz at a sound pressure level (SPL) of 100±3 dB(A) [27]. The noise was amplified using an AS 2000 amplifier (Taiwan) and played back using a pair of Microlab M563 speakers (Iran). The speakers were placed at equal distance from the four corners of the storage box and the sound pressure level was constantly controlled during the exposure period using a calibrated B&K 2245 sound level meter (Denmark).
Vibration exposure
The vibration exposure group was subjected to regular intervals of vibration for a duration of 60 minutes. A SVANTEK 583 whole body vibration meter (Poland/USA) equipped with a triple axis frequency analyzer was used to monitor the vibration acceleration. The vibrating mechanism was designed in a way as to ensure a dominant Z axis affective vibration acceleration of 1.2 m/s2 while keeping vibration along the other axes at a minimum level (less than the maximum allowed threshold of RMS 0.315 m/s2).
Lighting exposure
Light intensity during exposure was 1000 lux measured by Hanger EC1 lux meter (Sweden). The amount of light needed for testing was only provided through a projector equipped with 400watt metallic halide bulbs with white light. Remarkably, the lamp was installed upside the chamber and the mice were directly exposed to lighting at a distance of 50-cm.
Microwave exposure
The microwave exposure group was subjected to microwaves at a frequency of 850 to 960 MHz with a modulation frequency of 100 to 200 kHz and an output power density of 5 watts [28]. Holadays HI-1501 Microwave Survey Meter (USA) was used for this purpose. The radiation-emitting antenna of this device was placed above the center of the storage box as to ensure an equal degree of radiation dissipation.
Sperm analysis
Sperm parameters such as sperm count (106/mL) and sperm motility were analyzed manually. For this purpose, the epididymis tail was separated by making an incision and then placed in a phosphate buffer solution which was kept at 37 °C. In order to further facilitate the extraction of sperm from the epididymis tail, the samples were segmented using forceps. After this step, the samples are placed on a warm surface for 15 to 30 minutes as this increases sperm movement and eases the counting of sperm and morphology assessment. Sperm counting was done using a microscopic slide and neubauer haemocytometry slide. A drop of the sample solution is taken using a micropipette and placed between the microscopic slide and the Neubauer slide. Then the sample is placed under Olympus AH2 microscope (Japan) at 400× magnification and the sperm count is performed.
In order to manually measure sperm motility, a simple scaling system is used without the need for sophisticated tools. At least 5 microscopic fields must be studied systematically in order to categorize 200 sperm. The motility of the studied sperm is categorized as follows:
Percentage of progressive motile sperm.
Percentage of non-progressive motile sperm.
Percentage of immotile or static sperm.
The shape of the sperm is analyzed via colorization using Diff-Quik™ staining set. Normal sperm should have no abnormalities at the head, neck and tail while abnormal sperm will have deformed tails, no heads, two heads or microcephaly of the head [29].
Data analysis
Data was statistically analyzed using SPSS v20.0 (SPSS Inc., Chicago, Ill., USA) with descriptive statistics presented as mean, standard deviation and range. The Shapiro test was used to determine the normality of the data distribution. The one-way analysis of variance (ANOVA) test was used to compare mean sperm parameters in the studied groups. Dunnett’s test was used to compare the mean sperm parameters of each exposure group with the control group. The effect size of each type of exposure on the sperm parameters was determined using univariate analysis of variance. A significance level of 0.05 was used in this study.
Results
(Table 1) presents mean and standard deviation for all sperm parameters in the control group and the various exposure ones. The results show that the mean difference for all studied sperm parameters among the various exposure groups is statistically significant (P=0.001). The lowest sperm count belonged to the microwave exposure group (3.16±0.55 106/mL) and the highest sperm count belonged to the lighting exposure group (4.12±0.79 106/mL). In case of percentage of progressive motile sperm, it was found that all exposure groups had a lower mean compared to the control group (64.76±0.89 %). The lowest percentage of progressive motile sperm belonged to the noise exposure group (31.41±1.28 %) and the highest belonged to the lighting exposure group (60.67±0.77 %). The highest percentage of non-progressive motile sperm belonged to the lighting exposure group (34.01±1.51 %) and the lowest belonged to the vibration exposure group (31.88±1.26 %). The highest percentage of immotile sperm belonged to the microwave exposure group (36.58±1.24 %) and the lowest belonged to the lighting exposure group (5.31±1.66 %). The highest percentage of normal morphology belong to the vibration exposure group (74.11±0.64 %) and the lowest belong to the lighting exposure group (45.50±2.15%).
The mean sperm count in all exposed groups was lower than the ones of the control group (4.51±0.22 106/mL). The mean percent of sperm progressive motility in all exposed groups was lower than the ones of the control group (64.76±0.89 %). The percentage of sperm non-progressive motility was higher among the exposure groups compared to the control group (26.12±1.72%). The percentage of immotile sperm was higher among the exposure groups compared to the control group, except for lighting one (9.11±2.22 %). The percentage of normal morphology was lower in the exposure groups compared to the control group (72.56±1.52%)
According to (Table 2), the results of Dunnett’s test show significant difference in terms of sperm count between the control group with noise and microwave exposure group (P=0.001). The sperm count of the noise and microwave exposure group was 1.28 and 1.35 units lower than the control group, respectively. The mean difference of motility parameters between the control group and all of the exposure ones was statistically significant (P<0.01). The significant difference in terms of normal morphology between the control group with noise, lighting and microwave exposure group (P<0.01).
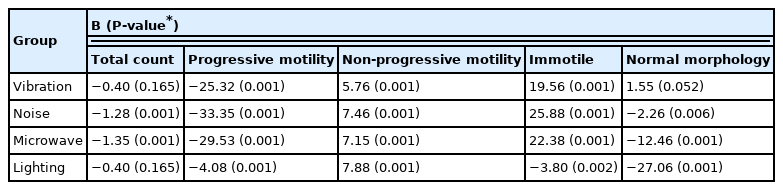
The results of the one-way variance analysis used to determine the effect size of each exposure type on sperm parameters are presented in (Table 3). The microwave exposure had the largest significant effect size for sperm count (B=−1.35; P= 0.001). In case of exposure to microwave, the chance that the sperm count will be decreased comparing to the control group is 1.35 times. The noise exposure had the largest significant effect size for progressive motility (B=−33.35; P= 0.001). In case of exposure to noise, the chance that the progressive motility percent will be decreased comparing to the control group is 33.35 times. The lighting exposure had the largest significant effect size for non-progressive motility (B=7.88; P= 0.001). In case of exposure to lighting, the chance that the non-progressive motility percent will be increased comparing to the control group is 7.88 times. The noise exposure had the largest significant effect size for immotile percent (B=22.38; P= 0.001). In case of exposure to noise, the chance that the immotile percent will be increased comparing to the control group is 22.38 times. The lighting exposure had the largest significant effect size for normal morphology (B=−27.06; P= 0.001). In case of exposure to lighting, the chance that the normal morphology percent will be decreased comparing to the control group is 27.06 times.
Discussion
The aim of this study was to assess sperm parameters including sperm count, sperm motility, and sperm morphology in mice after exposure to four hazardous occupational agents (noise, vibration, lighting and microwave).
Overall, the results show that sperm count, percent of progressive motility sperm and normal morphology were significantly reduced in all exposure group comparing to the control group. In addition, the percent of immotile sperm and non-progressive motility in the all exposure groups was significantly higher comparing to the control group.
One of the physical agents investigated in the present study was microwave radiation. This kind of radiation can disrupt the spermatogenesis process and reduce the fertility of the sperm. The results of the present study confirm it and also, indicate that microwave, as an inducing element, can affect the motility, morphology and total count of the sperm and therefore create changes in spermatogenesis and consequently endanger fertility. The results of the present study show that the mean difference of sperm parameters in microwave exposure group had a significant difference with the ones of control group, and also microwave exposure had the largest significant effect size on sperm count among the studied groups. The results of a study carried out by an infertility clinic regarding the effects of microwave exposure on sperm parameters showed that exposure to electromagnetic radiation can cause an increase in the number of abnormally shaped sperm and reduce sperm motility [30], which agrees with the findings of the present study. The results of Dasdag et al. regarding the effects of whole-body exposure to microwave radiation emitted by cellphones on sperm count in rats showed no significant difference in sperm count among the exposure group and the control group [24]. In another study, Kesari et al. (2010) assessed the effects of electromagnetic fields (EMFs) on testicular performance in Wistar rats. The results showed sperm morphology being more normal among the group exposed to 900MHz fields and concluded that exposure to EMFs caused an increase in testosterone levels, which led to a more normal sperm morphology [31]. The findings of Kesari et al. and Dasdag et al. did not agree with the present study, which may be due to differences in frequency, type of test animal and exposure duration. However, the results of a study conducted on the effects of electromagnetic fields on spermatogenesis showed that exposure to these fields causes reduced sperm mobility [32] which is in accordance with the present study.
Exposure to noise can induce stress and disrupt the synthesis and release of sex hormones such as testosterone, which can lower the production of sperm, and other sex hormones. This reduction can be the main cause of changes in the testicular tissue, which is due to the increase in the concentration of the luteinizing hormone (LH) (the reduction of testosterone in the noise exposure group is probably the reason for reduced serum LH concentration). The increase in cortisol levels due to noise exposure can induce negative effects on the synthesis or testosterone, spermatogenesis and steroidogenesis in the testes. Studies have shown that chronic cortisol elevation can cause reduced steroidogenesis in the testicular tissue [33]. The results of the present study confirm that noise exposure can adversely affect sperm parameters. The results of the present study indicate that all the sperm parameters in noise exposure group had a significant difference with the ones of control group, and also noise exposure had the largest significant effect size on percent of progressive motility and immotile sperm count among the studied groups. A study by Abbate et al. showed that exposure to noise caused a reduction in sperm count [34], which agrees with the findings of the present study. Moreover, the study by Jalali et al. (2012) regarding the effects of noise exposure on sperm motility revealed that exposure to noise during a full spermatogenesis cycle causes a significant reduction in sperm motility [33]. Another research concluded that exposure to noise had reduced the concentration of testosterone, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) suggesting that noise can lead to reduced sperm motility, which agrees with the findings of the present study [35].
Vibration exposure can have negative effects on the secretions deriving from the epididymis wall tissue, preventing the sperm from maturing and reducing their motility [36]. Therefore, vibration exposure can affect sperm parameters. The results of the present study show that all the sperm parameters in noise exposure group had a significant difference with the ones of control group, except for total count and normal morphology. The study by Saeed et al, in 2018 however, showed no significant difference between the sperm count of the vibration exposure group and the control group [37]. The study by Penkov et al. regarding the effects of whole-body vibration on sperm morphology indices showed that vibration can lead to reduced sperm count [14], which agrees with the findings of the present study.
Exposure to lighting can change the redox state of the sperm cell, which is accompanied by induced production of Reactive oxygen species (ROS). Changes in ROS play a vital role in the controlling of sperm movement and sperm fertilization capacity in mammals. Therefore, lighting exposure can affect sperm parameters by causing these changes [38]. The results of the present study indicate that all the sperm parameters in lighting exposure group had a significant difference with the ones of control group, except for total count and also lighting exposure had the largest significant effect size on percent of normal morphology among the studied groups. A study by Brandt et al. regarding the effects of artificial lighting (300 to 350 lux) on the semen quality of adult boars showed that the volume of semen and the overall number of motile sperm was lower in the exposure group compared to the control group [18]. Sayed et al. (2018) conducted a study on the effects of red, yellow, green, blue and white LED lights on testosterone concentration and sperm quality among roosters [39]. Their results showed that green light prevents the growth of the testes and has negative effects on almost all sperm characteristics monitored in their study. They also found that the green and blue exposure groups have a considerably lower testosterone level compared to the control group, suggesting that light can affect sperm quality, which is in agreement with the findings of the present study.
Workers are at risk of many different hazardous physical agents at the workplaces and each of these factors can have detrimental physical and psychological health consequences depending on the nature and the conditions of exposure. On the other hand, the health of the sperm is a necessary factor for fertility and any endogenous or exogenous cause can lead to it being damaged and become infertile. This makes the study of influential factors on sperm parameters ever more important. Based on the results of the present study, it can be said that noise, vibration, lighting, and microwave can cause changes in different sperm parameters, however for definite conclusion; more laboratory and field studies are required in this regard.
Conclusions
The results of the present study clearly show that exposure to noise, vibration, lighting and microwave can have an adverse effect on sperm count, sperm motility and sperm morphology. Among the four exposure scenarios, exposure to microwave has had the greatest effect on sperm count. Exposure to noise has had the greatest effect on progressive motility and immotile percent and exposure to light has had the greatest effect on normal morphology and non-progressive motility. Investigating the exposure to different levels of noise, vibration, lighting and microwave radiation are suggested for future lab and field studies for a better understanding of this issue. The limitations of the present study include the short exposure duration, problems related to working with lab animals and maintaining environmental conditions inside the storage containers of mice during the exposure period. Manual assessment of sperm parameters is another limitation of the present study, so it is recommended for obtaining a better test reliability, the all-semen analysis have done by the computer-automated semen analyzer. Moreover, histopathology of testis is needed to assure the effect of spermatogenesis which is ignored at the present study.
Acknowledgement
The author thanks to the Shahid Beheshti University of Medical Sciences for providing financial support.
Notes
Ethical statement
This study is approved by student research committee- Shahid Beheshti University of Medical Sciences (28-92/10/03).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT author statement
MBA: Conceptualization, Methodology, Investigation, Software, Writing-Original draft preparation; HM: Conceptualization, Methodology, Investigation, Data curation, Software, Supervision, Writing-Reviewing and Editing; SFD: Conceptualization, Methodology, Investigation, Supervision, Writing-Reviewing and Editing; FAB: Methodology, Investigation, Data curation, Writing-Reviewing and Editing; MAM: Methodology, Investigation, Software, Writing-Reviewing and Editing.
Informed consent
The study was approved by the ethical committee of school of public health & neuroscience research center- Shahid Beheshti University of Medical Sciences (Research ID. 28-92/10/03).