Experimental determination of indoor air concentration of 5-chloro-2-methylisothiazol-3(2H)-one/ 2-methylisothiazol-3(2H)-one (CMIT/MIT) emitted by the use of humidifier disinfectant
Article information
Abstract
A mixture of 5-chloro-2-methylisothiazol-3(2H)-one/2-methylisothiazol-3(2H)-one (CMIT/MIT) had been used as an active ingredient in humidifier disinfectants (HDs). Owing to its high reactivity, the atmospheric concentration of CMIT/MIT, following its use in HD, would be lower than expected assuming that it is removed by ventilation only. In order to evaluate the exposure concentration of CMIT/MIT used as an HD, room-scale chamber studies were conducted under plausible use of three different HD doses at air change rates (ACR) of 0.3, 0.5, and 1.0 h−1. Atmospheric CMIT/MIT was sampled using two serial impingers containing deionized water after the attainment of steady state. Water samples in which CMIT/MIT was dissolved were concentrated using a cosolvent evaporation method with efficiencies of 35.5 and 77.9% for CMIT and MIT, respectively. The estimated air concentration, assuming that all the CMIT/MIT is absorbed in deionized water, increased linearly with increasing emission rate, but was independent of the ACR. This indicates that the removal rate of CMIT/MIT via chemical reactions is more than the removal rate by ventilation. Further investigations on homogeneous and heterogeneous chemical reactions of CMIT/MIT under ambient conditions are necessary to understand the actual exposure concentration of the mixture in HD.
Introduction
A mixture of 5-chloro-2-methyl-4-isothiazolin-3-one/2-methyl-4-isothiazolin-3-one (CMIT/MIT) has been used as an active ingredient in humidifier disinfectants (HDs) [1–3]. Epidemiological investigations have shown that the use of CMIT/MIT as HDs resulted in HD-associated lung injury (HDLI), including interstitial pneumonitis and widespread lung fibrosis [3–5].
Isothiazolinones including a mixture of CMIT/MIT are classical multi-purpose biocides widely used in many consumer products [6–8]. They have been developed as active biocides because of their high reactivity with disulfide groups in proteins [9]. Reaction of the N-S bond of the isothiazolone ring with nucleophilic cells makes them active biocides [10]. It is well-known that isothiazolinones readily react with thiol-containing substances, such as glutathione [11]. Several studies on the stability of CMIT have also shown that they are degradable in water above pH 8.5 [8,12–15]. However, it is not understood how stable CMIT/MIT is after its release in the form of small aqueous aerosols from humidifiers. Previous study on the estimation of steady exposure level of CMIT/MIT used in a HD revealed that it was less stable in indoor environment than other conservative active ingredient of HDs, such as guanidine oligomers [16]. Electrophilic nature of CMIT/MIT is the likely cause of its low stability in air. Furthermore, its strong reactivity makes it difficult to assess human exposure to CMIT/MIT after its use in HDs. In addition, its high reactivity also poses a question regarding its reported effective concentrations causing toxicity to laboratory animals because current standard protocol does not entail direct sampling and analysis of the active ingredients released in the form of small aqueous aerosols [17].
To address the questions on the exposure concentration of CMIT/MIT after its use in HDs, a series of chamber experiments with varying emission rates from a humidifier as well as air change rate (ACR) were conducted. Atmospheric CMIT/MIT was sampled using two serial impingers in three different HD doses, with ACRs ranging from 0.3 to 1.0 h−1. Assuming 100% sampling efficiency, steady-state concentrations of CMIT/MIT were estimated. By comparing concentrations obtained by experiments with those predicted by assuming that CMIT/MIT is non-reactive, the relative importance of the reaction rate constant over the ACRs was compared.
Materials and Methods
Reagents
Analytical-grade CMIT (98.4%) was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany) and MIT (98%) was purchased from Sigma–Aldrich (St Louis, MO, USA). For preparing humidifier aerosols, a commercial product used as an active ingredient in HDs obtained in 2011 and stored at 4°C was used. According to the manufacturer, this product contained 72–77% water, 1–2% CMIT, 0.2–0.6% MIT, and 21–25% preserving agent (21–25% magnesium nitrate and 0.5% magnesium chloride). The CMIT and MIT concentrations in this product, measured using high performance liquid chromatography coupled with a UV detector (HPLC-UV), were 14,300 mg L−1 and 4,230 mg L−1, respectively.
Experimental chamber
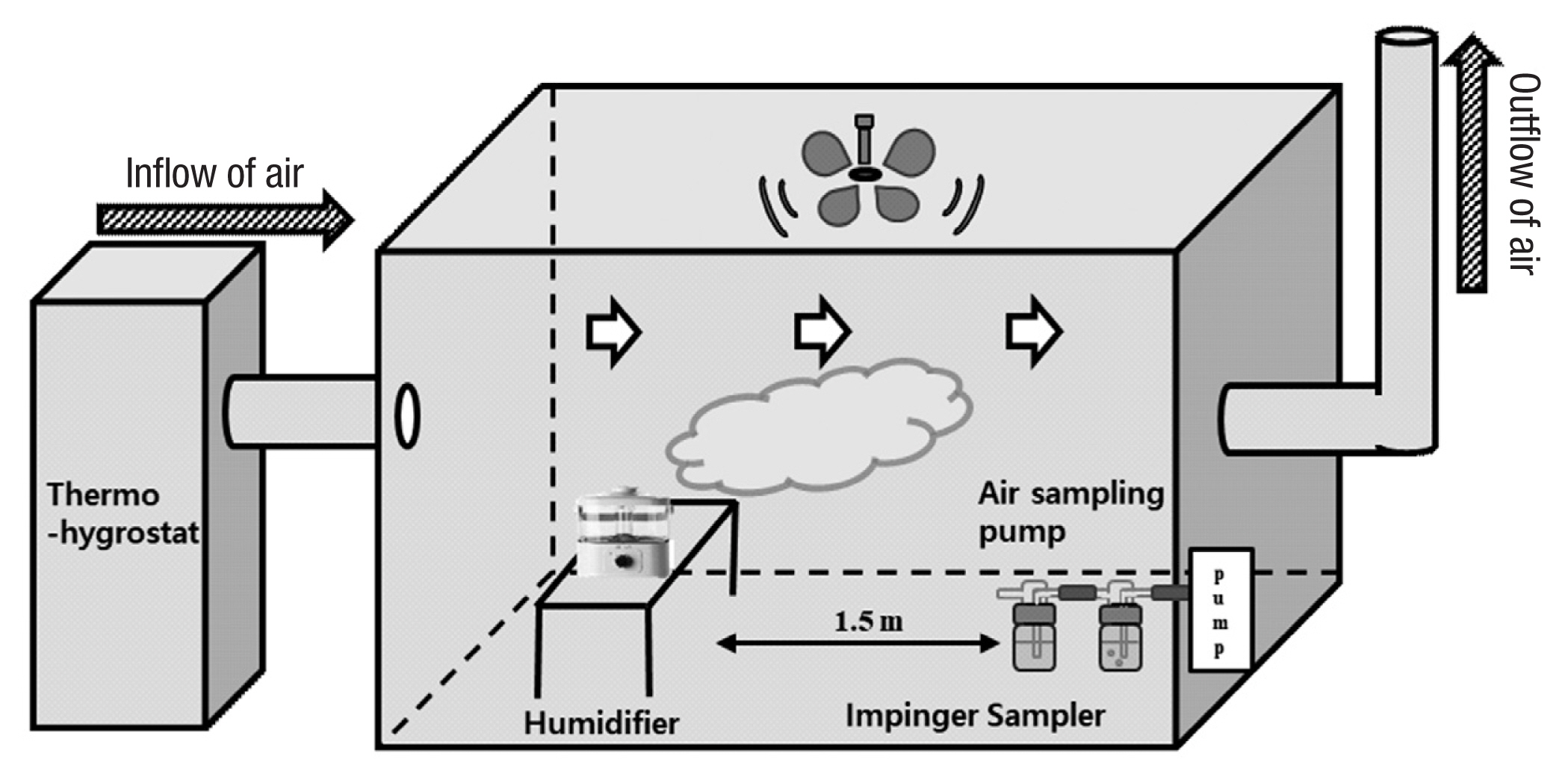
A custom-built 30.0 m3 stainless steel experimental chamber was used to measure the atmospheric concentration of CMIT/MIT after its release by the humidifier. The stainless chamber met the ASTM (American Society for Testing and Materials), US-EPA (US Environmental Protection Agency), and Greenguard standards [18–20]. The chamber received filtered air from a carbon filter and a high efficiency particulate air (HEPA) filter with a constant humidity and temperature. The ACR could be adjusted in the chamber by controlling the amount of air via heating ventilation and air conditioning (HVAC) system. The HVAC system was set to incoming air volume of 9 m3 for 0.3 h−1 ACR, 15 m3 for 0.5 h−1 ACR, and 30 m3 for 1 h−1 ACR. The chamber air temperature and humidity were monitored at 23±1°C and 50±3%, respectively. Each ACR was tested via a CO2 reduction method. Moreover, temperature and humidity were tested with an SPS sensor every 10 seconds. Figure 1 illustrates the schematic design of the experimental chamber used.
Humidifier
A DPI2000JH humidifier was purchased from Duplex (Ansan, Republic of Korea). The humidifier has a 5 L water tank and operates ultrasonically at a maximum spray capacity of 0.40 L h−1.
Chamber studies
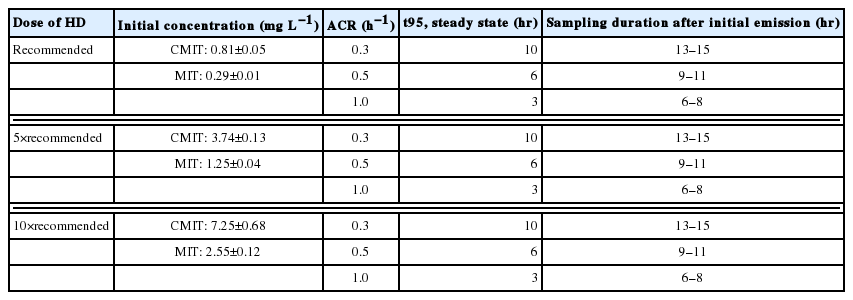
In order to evaluate human exposure, steady-state atmospheric concentrations of CMIT/MIT released from a humidifier were evaluated at nine use conditions (Table 1). Specifically, three ACRs (i.e., 0.3, 0.5, and 1.0 h−1) and three doses of HD were used. The doses of HD were that recommended by manufacturers (i.e., 10 mL HD in 2–3 L water), and five- and ten-times the recommended dose. The overall range of doses was the same as that used in a previous study [16]. Concentrations of CMIT and MIT in water tank were measured using HPLC method before each exposure experiment (Table 1). The measured concentration ratios were approximately 3:1 as the mass ratio of CMIT:MIT manufactured. Emission rate of CMIT/MIT was then calculated by multiplying the concentration and the humidifying rate which was measured for each experiment.
Assuming that the air in the experimental chamber is completely mixed, the atmospheric concentration (C) after the constant release is given by
where E is the emission rate (mg h−1), λ represents the ACR (h−1), k is the overall first-order elimination constant (h−1), V is the volume of the chamber (30 m3), and t is the experimental time (h). If we further assume that CMIT and MIT are non-reactive (i.e., k=0), Equation 1 is simplified to
Time required to attain the 95% steady-state atmospheric concentration (t0.95) is estimated as
where Q is the volumetric flow rate to the chamber (m3 h−1).
The amount and concentration of the actual HD used is measured to predict the air concentration, as mentioned previously. We assumed that CMIT/MIT is conserved and the water sampling efficiency is 100%. Sampling amount is calculated by multiplying sampling time, sampling rate, and predicted chamber concentration.
Sampling, pretreatment, and estimation of atmospheric CMIT/MIT concentration
In order to sample CMIT/MIT from the air in the chamber, a sampling method using two serial impingers was used [16]. The sampler was placed 1.5 m downstream of the humidifier in the test chamber (Figure 1). Chamber air was sampled using HPLC grade water. The air sampling rate was set to 7.5 L min−1 based on the average of the adult inhalation rate in Korea [21] and controlled using a flow regulator. Sampling was performed for 2 hours after the apparent steady state (3/λ). Samples collected from each impinger that were rinsed with water were transferred into an amber bottle and stored in a refrigerator (4°C) until analysis. Storage time did not exceed one week.
CMIT/MIT dissolved in water was extracted via a cosolvent method, because CMIT/MIT is water soluble and has shown a low recovery with other methods [7,22]. Sample and methanol, 100 mL each, were mixed in a round bottom flask; the solution was evaporated at 55°C using a rotary evaporator until approximately 50 mL of it remained. After further addition of 100 mL methanol, the solution was evaporated until 5 mL of it remained. The concentrated sample was transferred to a vial and the remaining solvent was evaporated under a gentle nitrogen stream at 55°C. Immediately after the complete evaporation of the solvent, the dried residue was dissolved in 200 μL acetone and this solution was subjected to gas chromatograph-mass spectrometry (GC-MS) analysis. Atmospheric concentration of CMIT/MIT was obtained from the extracted sample mass. It was calculated by dividing the measured concentration by the enrichment factor and extraction efficiency and by multiplying the impinge sample volume.
Instrumental analysis
Determination of CMIT/MIT in the product
Contents of CMIT and MIT in the commercial active ingredient of HDs were quantified using an HPLC system equipped with a Waters 515 pump (Waters, Milford, MA, USA), a Waters 717+ auto sampler, and a Waters 2998 photodiode array detector. Analytes were separated using a Fortis C18 (150 mm×4.6 mm; particle size of 5 μm) column (Fortis Technologies Ltd., Cheshire, UK) under isocratic conditions (30:70, methanol: water, v/v) at a flow rate of 1.0 mL min−1. The injection volume was 10 μL and both chemicals were monitored at 280 nm. This measured concentrations of CMIT/MIT in the product were used for the calculation of predicted concentrations.
Gas chromatographic determination of CMIT/MIT
Acetone aliquots containing CMIT and MIT after extraction were quantified using an Agilent 7890A gas chromatograph (GC) and 5975C mass spectrometry detector (MSD) (Agilent Technologies, Santa Clara, CA, USA). Separation was achieved using an Agilent DB-5 MS capillary column (30 m×0.25 mm; film thickness of 0.25 μm). Helium was used as the carrier gas at a constant flow rate of 1 mL min−1. The GC oven temperature was programmed to increase from 60°C (held for 1 minute) to 150°C (held for 4 minute) at 10°C min−1 and then to increase to 280°C (held for 2 minute) at 60°C min−1. The injection volume was 2 μL in a splitless mode. The MSD was operated in the electron ionization (EI) mode (70 eV). The injection port temperature was 250°C and was run in selective ion monitoring (SIM) mode. The selected mass-charge ratios (m/z) were 115 and 87 for MIT and 149 and 85 for CMIT.
Quality assurance and quality control
Instrumental detection limits (IDL) were determined by the error distribution method [23]. The IDL was calculated by multiplying the standard deviation of the measurements obtained by seven repeated analyses of the lowest standard with t distribution value of 3.143 (98% confidence interval, degree of freedom=6). Extraction recovery of the cosolvent evaporation method was assessed using 1.00 and 10.0 μg L−1 aqueous solutions prepared by dissolving CMIT and MIT in deionized water. Measurements were conducted in triplicate for each initial concentration.
Method detection limit (MDL) was the minimum concentration of the substance to be measured by the cosolvent method. MDL was determined by multiplying the standard deviation of the measured value obtained by six times with t value of 2.571 (98% confidence interval, degree of freedom=5), taking into account the 2–10 times the spike of the IDL concentration and recovery.
Results and Discussion
Quality control
The measured IDLs of CMIT and MIT were 2.17 and 3.99 μg L−1, respectively. The extraction recoveries using the cosolvent evaporation method were calculated using solutions at 1.00 and 10.0 μg L−1 (in triplicate); the recoveries were 35.5±8.8 and 77.9±22.2% for CMIT and MIT (n=6), respectively. The lower recovery of CMIT than that of MIT is likely attributed to its higher Henry’s law constant [24]. Values of MDL were estimated as 0.29 and 0.14 μg L−1 for CMIT and MIT (n=6), respectively.
Estimation of atmospheric concentration of CMIT/MIT
Table 2 summarizes the estimated atmospheric concentrations of CMIT/MIT, assuming that they are non-reactive in the chamber under all the nine experimental conditions with different doses of HD and ACRs. As shown, the estimated air concentrations of CMIT/MIT based on experimental measurements increased linearly with increasing HD dose. This indicates that the assumption of complete mixing is satisfactory. It is also notable that the estimated concentrations based on experiments were much lower than those predicted, assuming that there were no chemical reactions and that they were almost independent of the ACR. This supports the fact that the concentrations of CMIT/MIT in the test chamber are determined by reactive removal and the corresponding pseudo-first order reaction rate constant would be greater than the highest λ, 1.0 h−1.
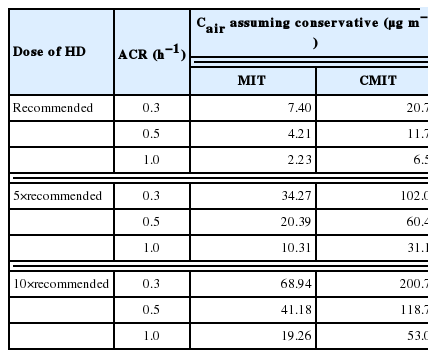
Atmospheric concentration of 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT) and 2-methyl-4-isothiazolin-3-one (MIT) estimated from theoretical calculations, assuming that they are conserved and the amount of CMIT and MIT measured using chamber studies
Considering the vapor pressure of CMIT (2.39 Pa) and MIT (0.083 Pa) [25], they are likely to exist in a gaseous state after their release in the form of aqueous aerosols at a relative humidity of 50%. To our best knowledge, for air, no reaction rate constants for CMIT/MIT have been reported. Therefore, their evaluation under typical indoor environments is urgently required. Reactions of CMIT/MIT with atmospheric oxidants, such as OH radical should be important. According to AOPWin program, the half-lives of CMIT and MIT at typical outdoor OH radical concentrations are predicted to be 5.8 and 4.8 h, respectively [26]. The corresponding photodegradation rate constants are much lower than the overall elimination rate constants (>1.0 h−1) obtained in this study, indicating that the oxidative degradation of CMIT and MIT might not be the major chemical degradation pathways, although these predictions have high uncertainties. Heterogeneous reactions with particulate matter and indoor materials could explain this discrepancy [9,12]. Therefore, further studies are needed to evaluate CMIT/MIT reactions with various materials in indoor environments in Korea.
Implications for assessing retrospective risks of CMIT/MIT as humidifier disinfectants
CMIT/MIT have been used in several consumer products such as shampoos, lotions, and liquid soaps. In Europe, contact dermal exposure to CMIT/MIT has been of significant concern [27,28]. In addition to dermal exposure via direct contact, cases of concern with respect to airborne products (e.g., paint) have been reported with some patients exhibiting asthmatic symptoms [27,29]. Limited inhalation toxicity studies using rats have shown lung redness and inflammation at high doses [30,31].
However, it should be noted that these toxicity studies have reported atmospheric concentration of CMIT/MIT not from direct measurements. When a commercial product was used, test substances also contain non-volatile solutes such as magnesium salts. The concentration of CMIT/MIT would have been calculated from the mass of aerosols trapped in the filters, assuming that the fraction of the active ingredient in filtered residues is the same as that in the initial test substance. CMIT/MIT is suspected to be highly reactive in air; thus, the reported dose of CMIT/MIT in toxicity tests could have been overestimated. Adverse effects of CMIT/MIT on organisms are directly related to their atmospheric concentrations. Without knowing the behavior of CMIT/MIT in living environment or the test systems for inhalation toxicity, the reliability and applicability of the reported no-effect concentrations in inhalation toxicity tests would be of limited use. Although a refined risk assessment under HD use conditions suggested that the estimated health risks by CMIT/MIT could be only expected at the highest HD doses [16], a comparison of the experimentally measured and calculated exposure concentrations, assuming no chemical reactivity, might lead to an underestimation of the risks involved. In the absence of the inhalation toxicity endpoints obtained using experimentally measured doses, the best and most conservative approach for health risk assessment would be the estimation of the exposure concentration using a well-mixed indoor air model.
Conclusion
Exposure concentrations of CMIT/MIT used in HD were assessed in a room-scale chamber study under plausible use scenarios at three ACRs, 0.3, 0.5, and 1.0 h−1. The estimated air concentrations assuming 100% absorption in deionized water increased linearly with increasing emission rate but was independent of the ACR. The removal of CMIT/MIT due to chemical reactions was higher. This indicates that the actual exposure concentration of CMIT/MIT would be highly variable among HD consumers, requiring further investigations on homogeneous and heterogeneous chemical reactions of CMIT/MIT under ambient conditions.
Acknowledgment
This study was supported by the National Institute of Environmental Research and the Ministry of Environment.
Notes
CRediT author statement
SKP: Methodology, Data Curation, Writing – Original Draft; HSS: Data Curation, Resources; HJP: Data Curation; YSK: Data Curation; SHR: Conceptualization, Writing – Reviewing & Editing; JK: Conceptualization; SK: Conceptualization; JHL: Conceptualization, Funding Acquisition, Writing – Reviewing & Editing; JHK: Conceptualization, Methodology, Supervision, Writing – Reviewing & Editing