Study on the biodegradation of perfluorooctanesulfonate (PFOS) and PFOS alternatives
Article information
Abstract
Objectives
In this study, we investigated the biodegradation features of 4 perfluorooctanesulfonate (PFOS) alternatives developed at Changwon National University compared to those of PFOS.
Methods
Biodegradation testing was performed with microorganisms cultured in the good laboratory practice laboratory of the Korea Environment Corporation for 28 days following the Organization for Economic Cooperation and Development guidelines for the testing of chemicals (Test No. 301 C).
Results
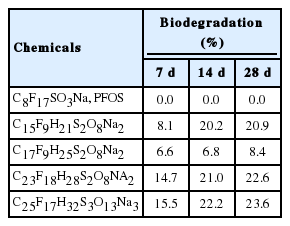
While C8F17SO3Na, PFOS sodium salt was not degraded after 28 days, the 4 alternatives were biodegraded at the rates of 20.9% for C15F9H21S2O8Na2, 8.4% for C17F9H 25S2O8Na2, 22.6% for C23F18H28S2O8Na2, and 23.6% for C25F17H32O13S3Na3.
Conclusions
C25F17H32S3O13Na3, C23F18H28S2O8Na2, and C15F9H21S2O8Na2 were superior to PFOS in terms of biodegradation rates and surface tension, and thus they were considered highly applicable as PFOS alternatives. Environmental toxicity, human toxicity, and economic feasibility of these compounds should be investigated prior to their commercialization.
Introduction
Perfluorooctanesulfonic acid (PFOS), a perfluorinated compound, is a synthetic compound. Since PFOS has excellent qualities in terms of surface activity, water repellency, oil repellency, thermal resistance, chemical resistance, and rub resistance, it has been broadly applied broadly as a fire fighting foam, coating agent for items such as carpet,clothing, paper, and leather, commercial stain-preventing agent, metal cleaner, and aviation hydraulic fluids. As such, PFOS is of high value for both industrial and consumer uses; however, PFOS is not easily degraded and remains in the environment or the human body for a long period of time [1,2].
A cerebellar test in rats showed that PFOS caused neurotoxicity and decreased the survival rate of infant rats in pregnant rats, causing reproductive toxicity. A recent study reported 6.06 ng/mL as the mean blood concentration of PFOS in Koreans [3].
Thus, PFOS was listed as a persistent organic pollutant during the 4th Conference of the Parties in the Stockholm Convention held in Geneva, Switzerland, May 2009, and was officially announced on August 26, 2010 after public comment periods of the countries directly involved. In South Korea (hereafter Korea), the enforcement ordinance for the Persistent Organic Pollutants Control Act was revised on April 11, 2011, listing PFOS and PFOS salts as persistent organic pollutants and strictly limiting their production, export, and use.
Although 3M volutariliy suspended its PFOS production in 2002 and developed alternatives including perfluorobutanesulfonate with a reduced number of carbon chains, a fully replacable material for PFOS has not been developed. The present study investigated the biodegradation features of 4 types of PFOS alternatives developed by Vijaykumar et al. [4] and compared these molceules to PFOS.
Materials and Methods
Test Substances
Test substances for biodegradation testing included 4 types of PFOS alternatives synthesized in the laboratory of professor Dong Soo Shin at Changwon National University and included C15F9H21S2O8Na2 (molecular weight [MW], 610.42), C17F9H25S2O8Na2 (MW, 638.47), C23F18H28S2O8Na2 (MW, 884.54), and C25F17H32S3O13Na3 (MW, 1028.65) as well as C8F17SO3Na (MW, 522.11), a PFOS in sodium salt.
Test Methods and Microorganisms
Biodegradation testing was performed following the Organization for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals (Test No. 301C).
Microorganisms used for the test were collected from 10 sites in Korea such as municipal sewage treatment plants, industry waste water treatment plants, rivers, lakes, and the sea, and cultured in the good laboratory practice laboratory of the Korea Environment Corporation for at least 1 month.
Test Equipment
An OxiTop Control (OxiTop Control 100, WTW, Weilheim, Germany) was used to measure the biodegradation rate according to the test method in the OECD guidelines for the testing of chemicals (Test No. 301 C). The OxiTop Control measures biochemical oxygen demand (BOD) in real-time based on pressure changes within a tightly closed test bottle. The machine is composed of test bottles, measurement heads, a remote controller, and an agitation system.
Biodegradation Test
One type of PFOS sodium salt (C8F17SO3Na) and 4 alternatives (C15F9H21S2O8Na2, C17F9H25S2O8Na2, C23F18H28S2O8Na2, C25F17H32S3O13Na3) were subjected to biodegradation testing.
A total of 0.015 g each of the 5 test substances (C8F17SO3Na, C15F9H21S2O8Na2, C17F9H25S2O8Na2, C23F18H28S2O8Na2, C25F17H32S3O13Na3) was transferred into bottles #1 to #5, and then 150 mL of deionized water (DW) was added to each bottle.
For bottles #6 to #20, each of the 5 test substances (C15F9H21S2O8Na2, C17F9H25S2O8Na2, C23F18H28S2O8Na2, C25F17H32S3O13Na3) was added to make 3 replicates. To each test bottle, we added 0.015 g of each test substance (final concentration of test substance, 100 mg/L), 1.8 mL basic medium, 143.64 mL DW, and 4.56 mL microorganisms (concentration, 30 ppm; dry weight, 9.9 mg). To evaluate the conditions of the microorganisms, bottle #21 contained 0.015 g aniline (C6H7N, 99.6%; Sigma-Aldrich, St. Louis, MO, USA) (100 mg/L) as a control substance, 1.8 mL basic medium, 143.64 mL DW, and 4.6 mL microorganisms. In order to compare the biodegradation rates of the 5 test substances, bottle #22 contained 1.8 mL basic medium, 143.64 mL DW, and 4.56 mL microorganisms. These 22 prepared test bottles were placed on the agitation system after setting the remote controller and then incubated for 28 days. The internal temperature of the incubator was 24.9˚C to 25.1˚C and the pH was 6.70 to 7.92.
The biodegradation rate (%) of test substances was calculated as follows:
The BOD of the Test Substances was Calculated as Follows:
BOD (mg/L) of test substances = (mean BOD of 3 replicates of each test substance [e.g., BOD of bottle #6 + BOD of bottle #7 + BOD of bottle #8/3])-(each BOD of bottle #1, BOD of bottle #2, BOD of bottle #3, BOD of bottle #4, or BOD of bottle #5+(BOD of bottle #5+BOD of bottle #22))/concentration of each test substance
The BOD of the control substance was calculated as follows:
BOD (mg/L) of control substance = (BOD of bottle #21-BOD of bottle #22)/concentration of control substance
The theoretical oxygen demand (ThOD) ThOD of the test substances or control substance was calculated as follows:
CcHhFfNnNanaOoPpSs
ThOD (mg/L)=16(2c+1/2[h-f-3n]+3s+5/2p+1/2na-o)/MW
Isolation and Identification of Degrading Microorganisms
A mineral medium produced in the biodegradation laboratory of Chonnam National University was used as a medium for the isolation of microorganisms. The only carbon source in the medium was the PFOS alternatives used in the test. Microorganisms were cultured in a 35˚C incubator under aerobic conditions for 24 hours and visually inspected for proliferation, followed by sub-culture on solid media at least 5 times. The isolated microorganisms were subjected to DNA sequencing of the 16S rRNA genes for identification.
Results
The degradation rate of aniline, the control substance, was 73.7% on the 7th day and 83.6% on the 14th day, confirming the normal states of the microorganisms used in the study (if the degradation rate is 40% or more on the 7th day and 65% or more on the 14th day, the microorganisms were considered to be normal based on the criteria of the OECD guidelines for the testing of chemicals, Test No. 301 C). For C8F17SO3Na, the sodium salt PFOS, the mean BODs were 6.53, 17.60, and 30.57 mg/L on the 7th, 14th, and 28th days, respectively. The BODs of the group without test substance (blank) were 13.20, 24.10, and 35.80 mg/L on the 7th, 14th, and 28th days, respectively. When C15F9H21S2O8Na2 was tested, the BODs were 20.60, 42.70, and 54.97 mg/L on the 7th, 14th, and 28th days, respectively. The mean BODs of C17F9H25S2O8Na2 were 19.90, 31.10, and 44.53 mg/L on the 7th, 14th, and 28th days, respectively. When C23F18H28S2O8Na2 was added, the mean BODs were 18.83, 35.20, and 49.80 mg/L on the 7th, 14th, and 28th days, respectively. C25F17H32S3O13Na3 addition resulted in mean BOD values of 26.53, 43.10, and 56.30 mg/L on the 7th, 14th, and 28th days, respectively (Table 1).
When the degradation rates were calculated using the biodegradation calculating formula, C8F17SO3Na, PFOS sodium salt showed a negative value, indicating that no degradation occurred over the 28 days. The biodegradation rate of C15F9H21S2O8Na2 was 8.1%, 20.2%, and 20.9% on the 7th, 14th, and 28th days, respectively. The biodegradation rate of C17F9H25S2O8Na2 was 6.6%, 6.8%, and 8.4% on the 7th, 14th, and 28th days, respectively. In addition, the biodegradation rate of C23F18H28S2O8Na2 was 14.7%, 21.0%, and 22.6% on the 7th, 14th, and 28th days, respectively. Finally, the biodegradation rate of C25F17H32S3O13Na3 was 15.5%, 22.2%, and 23.6% on the 7th, 14th, and 28th days, respectively (Table 2).
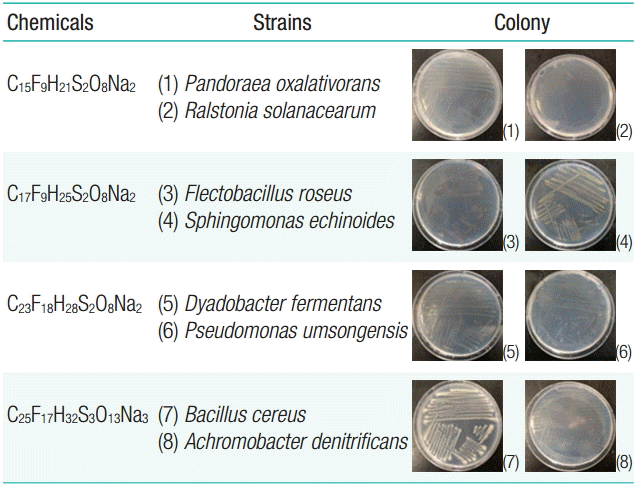
Microorganisms capable of degrading the 4 PFOS alternatives were isolated and identified based on their DNA sequences. The DNA sequences of C15F9H21S2O8Na2-degrading microorganisms showed 99% and 96% homology with those of Pandoraea oxalativorans and Ralstonia solanacearum, respectively. For C17F9H25S2O8Na2, the DNA sequences showed 99% homology with of the sequences of Flectobacillus roseus and Sphingomonas echinoides. Microorganisms degrading C23F18H28S2O8Na2 showed 100% of homology with both Dyadobacter fermentans and Pseudomonas umsongensis. Those of C25F17H32S3O13Na3 showed 99% homology with Bacillus cereus and Achromobacter denitrificans (Figure 1).
Discussion
C8F17SO3Na, the PFOS sodium salt, was not degraded by microorganisms over the 28-day experimental period. The biodegradation rates of C25F17H32S3O13Na3 and C23F18H28S2O8Na2, which have 3 times as many carbons but similar numbers of fluorines (17 and 18), were 22.6% and 23.6%, respectively, showing the highest degradation rates. The next highest biodegradation rate was 20.9% of C15F9H21S2O8Na2, which contained 15 carbon atoms and only 9 fluorine atoms. PFOS has superior features in surface activity, water repellency, and oil repellency because of its low surface tension. When surface tension was measured at the same concentration (500 mg/L) using a tensiometer (KSV Sigma 702, Biolin Scientific, Stockholm, Sweden), the surface tension of the 4 PFOS alternatives was 20.94 to 28.17 mN/m, whereas that of the PFOS sodium salt C8F17SO3Na was 46.18 mN/m. Taken together, the 3 substances (C23F18H28O8S2Na2, C25F17H32O13S3Na3, C15F9H21O8S2Na2), which showed biodegradation rates of higher than 20%, were determined to be highly applicable as PFOS alternatives. However, they should be investigated further to determine their environmental toxicity, human toxicity, and economic feasibility before commercialization as PFOS alternatives.
Acknowledgements
This study was funded by the Korea Environmental Industry and Technology Institute, the Ministry of Environment (KME, 412-111-008).
Notes
The authors have no conflicts of interest associated with material presented in this paper.