Interaction Between Bronchiolitis Diagnosed Before 2 Years of Age and Socio-Economic Status for Bronchial Hyperreactivity
Article information
Abstract
Objects
The prevalence of asthma has increased in recent decades globally. The objective of the present study is to elucidate whether hospitalization for bronchiolitis in infancy and low socioeconomic status interact for bronchial hyperreactivity during teenage years.
Method
We studied 522 children age 13-14 years attending schools in rural and urban areas to investigate the risk factors for bronchial hyperreactivity (BHR), defined as a provocation concentration of methacholine that causes a decrease of 20% (PC20) in forced expiratory volume within 1 second. Clinical examination, skin prick test, spirometry, and methacholine challenge were performed on all study subjects, who provided written consent. We used multivariate logistic regression to investigate the risk factors for BHR, and analyze the interaction between hospitalization for bronchiolitis in infancy and low socioeconomic status.
Results
Forty-six (10.3%) positive BHR cases were identified. In the multivariate logistic analysis, as independent predictors of BHR, adjusted odds ratio of bronchiolitis diagnosed before 2 years of age in low income families was 13.7 (95% confidence interval, 1.4 to 135.0), compared to reference group, controlling for age, gender, parental allergy history, skin prick test, and environmental tobacco smoke (ETS) exposure. Interaction was observed between bronchiolitis before 2 years old and low socioeconomic status on children's bronchial hyperreactivity (p-interaction=0.025).
Conclusions
This study showed that bronchiolitis diagnosed before 2 years of age and low socioeconomic status interacted on children's bronchial hyperreactivity. Prevention of acute respiratory infection in early childhood in low socioeconomic status is important to prevent BHR as a precursor of asthma.
INTRODUCTION
The prevalence of asthma has increased during recent decades all over the world, and it is still rising in developed countries. However, the economic and precautionary effects of asthma are likely greater in the developing world, where the prevalence is also rising [1]. Primary prevention strategies to combat the asthma epidemic are therefore urgently sought, but they must be based on a sound understanding of the various determinants of the onset of asthma. Nonspecific bronchial hyperreactivity (BHR) is an important feature of asthma, and its identification has been used in the research field to strengthen the assessment of possible asthma. Comparison of risk factor profiles for wheezing, asthma, and BHR could facilitate a better understanding of the mechanisms underlying these conditions as well as of their similarities and differences.
Bronchiolitis is defined as a lower respiratory infection (LRI) in infants usually caused by virus. Acute viral bronchiolitis is one of the most common causes of hospitalization during infancy. Wheezing is common in infants and young children who suffer from LRI caused by different viruses, such as respiratory syncytial virus (RSV), rhinovirus and so on. There is some evidence that early-life bronchiolitis may predispose some infants to the development of childhood asthma [2-4]. However, risk factors for later lung function abnormalities or for the persistence of BHR after wheezing in infancy have rarely been studied.
The objective of our study is to elucidate whether hospitalization for bronchiolitis in infancy and low socioeconomic status (SES) interact for bronchial hyperreactivity during teenage years.
MATERIALS AND METHODS
I. Study Subjects
We studied 522 children age 13-14 years attending schools in rural and urban areas to investigate the risk factors for BHR, defined as a provocation concentration of methacholine that caused a decrease of 20% (PC20) in forced expiratory volume within 1 second. Survey questionnaire, clinical examination, skin prick test, spirometry, and methacholine challenge were performed on only students, who provided written consent. Written consent was obtained from the parents of the subjects. Finally, 522 students completed survey questionnaire, 474 student performed BHT, and 449 students got skin prick test.
II. Skin Prick Test (SPT)
Subjects underwent SPT with a battery of 14 allergens including grass, tree, and weed pollens, dust mites (Dermatophagoides pteronyssinus and farinae), dog and cat epithelium, moulds (Aspergillus fumigates and Alternaria alternata), peanut, milk, egg, and bean (Allergopharma Co, Germany). The SPTs were performed on the forearm using an ALK-Lancet (ALK-Abello, Horsholm, Denmark). Negative and positive control tests were performed with 50% glycerine-saline and histamine (10 mg/mL), respectively. After 15 minutes, the skin prick weal induced by allergens and the negative and positive controls were outlined and copied onto permanent records with adhesive cellotape; the orthogonal diameters were subsequently measured. A positive SPT result was defined as a weal at least 3mm larger than the diameter of the negative control. All subjects avoided short-acting antihistamines for 72 hours, long-acting antihistamines for 5 days, and tricyclic antidepressants for 2 weeks before STP [5].
III. Methacholine Challenge Test (MCT)
After inhaling an initial control solution of isotonic saline solution, study subjects were administered doubling concentrations of methacholine, starting from 0.0625 mg/mL, up to 16 mg/mL (if no response), by a dosimeter . All the spirometries were conducted on a KoKo Pneumotach Spirometer (Susquehanna Micro, Inc.; Red Lion, PA, USA), as previously described [4]. The variables measured were forced vital capacity (FVC), forced expiratory volume (FEV)1, and mid-expiratory flow (FEF25-75). During the MCT, FEV1 values were measured 1 minute after the end of the corresponding inhalation. Each FEV1 measurement was expressed as a percentage from the baseline record. The provocation concentration of methacholine, defined as causing a decrease of 20% in FEV1 (PC20), was recorded. According to the standard protocol in our institute, all study subjects were instructed to stop any antiasthma medication before MCT. The washout period was 12 hours for short-term beta-agonists, 48 hours for long-term beta-agonists, and 7 days for inhaled corticosteroids. If the provocation concentration of methacholine, defined as causing a decrease of 20% in FEV1 (PC20), is less than 16mg/ml, it is diagnosed as a positive methacholine test.
IV. Statistical Analysis
Continuous variables were summarized by central tendency measures (mean or median), together with a dispersion measure such as standard deviation or range.
Categorical variables were described by their distribution of frequencies and percentages, both globally and in special interest subgroups. Comparison between groups was performed by using a Chi-square test and Fisher's exact test. We considered p-value of 0.05 as significant level.
Multivariable logistic regression model was used to investigate the risk factors for BHR. The odds ratios are shown with Wald's 95% confidence intervals (CI). Statistical analyses were done using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
The study subjects included 182 males and 340 females, with ages ranging between 13 and 14 years.
148 students (28.4%) have paternal allergy, 374 students (71.6%) have no paternal allergy. Some students did not provide the information about paternal education level (5 students) and family incomes (11 students). At final analysis, they are excluded. 1 student (0.2%) has a paternal education level of no years, 6 students (1.2%) have a paternal education level of less than 6 years, 29 students (5.6%) have a paternal education level of less than 9 years, and 231 students (44.6%) have a paternal education level of less than 12 years. 229 students (44.3%) have a paternal education level of education less than 16 years, and 21 students (4.1%) have a paternal education level of education more than 16 years. 180 students (35.2%) have family incomes of less than 1.5 million won monthly, 244 students (47.8%) have family incomes of 1.5-3.0 million won monthly, and 87 students (17.0%) have family incomes of more than 3.0 million won monthly.
The age in subgroup in the rural area (Pocheon) is from 13 years to 14 years old, while that in urban A and urban B in Seoul is 13 years old. Gender ratio is not different between the three subgroups. The rate of having a parental allergy is not different between the three subgroups. However, factors related to socioeconomic status including father's education level and family's incomes are significantly different (Table 1).
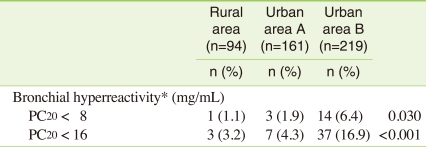
Positive BHR is defined as a provocation concentration of methacholine that causes a decrease of PC20 in forced expiratory volume in 1 second. When PC20 is less than 8 mg/dL, there are 18 (3.8%) positive BHR cases. When PC20 is less than 16 mg/dL, there are 47 (9.9%) positive BHR cases. Urban B area having low socioeconomic status shows the highest positive BHR rate at 16.9% (Table 2).
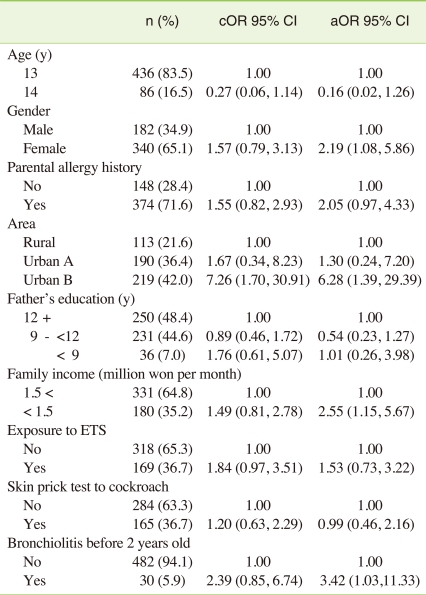
In the univariate logistic analysis, as independent predictors of BHR, crude odds ratio of bronchiolitis diagnosed before 2 years of age was 2.39 (95% CI, 0.85 to 6.74), compared to reference group who did not experience bronchiolitis diagnosed before 2 years of age. And crude odds ratio of low family income less than 1.5 million won per month, was 1.49 (95% CI, 0.81 to 2.78), compared to reference group who have family income more than 1.5 million won per month.
In the multivariate logistic analysis, as independent predictors of BHR, adjusted odds ratio of bronchiolitis diagnosed before 2 years of age was 3.42 (95% CI, 1.03 to 11.33), compared to reference group, controlling for age, gender, parental allergy history, skin prick test, and Environmental tobacco smoke (ETS) exposure. Adjusted odds ratio of low family income less than 1.5 million won per month was 2.55 (95% CI, 1.15 to 5.67), controlling for age, gender, parental allergy history, area, skin prick test, and ETS. Female children were also vulnerable group to bronchial hyperreactivity. Adjusted odds ratio of female children was is 2.19 (95% CI, 1.08 to 5.86) compared to male children, controlling for age, parental allergy history, bronchiolitis diagnosed before 2 years of age, area, skin prick test, and ETS exposure (Table 3).
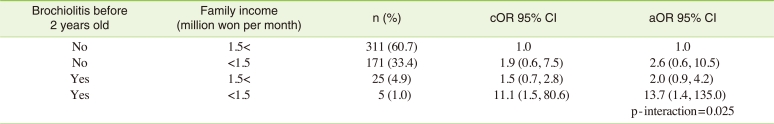
To investigate joint effect of bronchiolitis before 2 years old and low socioeconomic status on children's bronchial hypersensitivity, interaction between them was explored. In the multivariate logistic analysis, as independent predictors of BHR, adjusted odds ratio of bronchiolitis diagnosed before 2 years of age in low income families was 13.7 (95% CI, 1.4 to 135.0), compared to reference group, controlling for age, gender, parental allergy history, skin prick test, and ETS exposure (Table 4). Interaction was observed between bronchiolitis before 2 years old and low socioeconomic status on children's bronchial hyperreactivity (p-interaction=0.025).
DISCUSSION
Hospitalization for bronchiolitis in infancy has been linked with lung function abnormalities [4,6], reactive airways [7-9] and atopic asthma [10] until 10-13 years of age. Infants with wheezing severe enough to require hospitalization seem to be vulnerable groups with host-specific characteristics, which modify their responses to viral infections [11].
This raises the question of what are the host-specific characteristics of vulnerable groups? While the scope of modifying genetic influences is still limited, attention has focused on environmental factors that can be changed by feasible interventions. Thus, we should have interest on numerous influences as the potential causes for childhood asthma, including early allergen exposure, method of infant feeding, viral respiratory infection, environmental tobacco smoke exposure, and pet contact.
Asthma and bronchial hyperreactivity, after many nonsymptomatic years, have a tendency to recur in early adulthood [12]. Therefore, minor changes in lung function or in bronchial reactivity, though clinically nonsignificant, may be useful predictors of subsequent morbidity. Our study found that bronchiolitis diagnosed before 2 years of age in low income families can be an important risk factor of bronchial hypersensitivity. In that point, we found that bronchiolitis diagnosed before 2 years of age and SES , such as low income families, interact to modify bronchial responsiveness mutually. We established regression analysis to validate their interaction. Our study is unique in demonstrating the interaction between bronchiolitis diagnosed before 2 years of age and SES statistically. Our study has some limitations. This study is cross-sectional, not a cohort study. Viral infections were not studied by antigen detection in the nasopharyngeal aspirates obtained during hospitalization and antibody determination in sera in this study. There may be recall bias in our study. But, we checked closely whether bronchiolitis was diagnosed by doctor, and bronchiolitis, usually caused by respiratory syncytial virus (RSV) and rhinovirus infection, is an important cause of severe lung disease in infants, it is less likely that recall bias may be involved in this study.
There are increasing evidence suggests that it is immunologically mediated. Experiments in mice suggest that this may be due to differential T-cell activation producing either type 1 or type 2 cytokines. This hypothesis is also supported in patients with severe RSV bronchiolitis, by measuring messenger RNA (mRNA) for interleukin-4 (IL-4) and interferon-gamma (IFN-gamma), by polymerase chain reaction, in nasopharyngeal aspirates (NPAs) and bronchoalveolar lavage (BAL) fluids. These findings provide the first direct evidence in infants that in RSV bronchiolitis there are divergent T-cell responses and suggest that more than one mechanism may be responsible for immune-mediated disease enhancement [13].The combination of atopic heredity and elevated cord blood IgE resulted in the best predictive discrimination with regard to the development of allergic disease [14].
In the study of Wennergren et al., 37% of <24-month-old children hospitalized for bronchiolitis had abnormal results in histamine challenge at the age of 10 years, the figure being between our BHR values obtained by two measures, 26% for exercise and 46% for the methacholine challenge, at an average of 12.3 years of age. Wennergren et al did not find any association between early atopy and asthma or BHR at the age of 10 years [10]. In our study and another study [15], instead, the positive skin prick test was a significant predictor of activity teenagers. Atopic asthmatic schoolchildren have greater hyperreactivity to methacholine than nonatopics [16]. Our findings are in accordance with a recent birth cohort study from Germany [17], in which BHR at school age was significantly associated with sensitization to perennial allergens in early life, particularly in those children who suffered from current wheezing.
Families with low socioeconomic status are susceptible to numerous environmental hazards [18-20].
In a meta-analysis including 22 studies, maternal smoking was associated with small but statistically significant deficits in lung function in school-aged children [21]. The prevalence of smoking and nicotine addiction among parents of children with asthma or bronchiolitis who bring their children to a pediatric emergency department is high [22].
In our study, we observed increased adjusted odds ratio of current ETS exposure on BHR at late school age, which was not significant statistically. Although there is some evidence that in utero exposure to tobacco smoke may increase bronchial responsiveness continuing through childhood [23], many factors, such as early-life passive smoking and later active smoking, complicate the interpretation of the results [20,24]. A recent Swedish long-term follow-up study after bronchiolitis in infancy stressed the link between early-life tobacco smoke exposure, later BHR, and later asthma [25]. Early passive smoking was an independent risk factor for BHR and for active smoking at young adult age, and both were associated with chronic asthma [22].
Taking all these things into consideration, ETS operates as a cofactor with other insults such as intercurrent infections as a trigger of wheezing attacks, rather than as a factor that induces asthma, whereas in utero exposure acts to increase physician-diagnosed asthma.
Carroll et al. [26] reported a negative association between increasing birth weight and bronchiolitis diagnosis. LBW is associated with higher risk of being hospitalized for bronchiolitis in infancy [27].
People with low SES are more vulnerable to air pollution than others. They are exposed to infection, nutritionally deficient, and often lived in more polluted area.
Nutrition status can be acting as a effect modifier between exposures to airborne particulate matter and asthma [28,29]. Earnst et al. reported that among factors potentially linked to SES, the presence of a cat at home (OR, 1.63; 95% CI, 1.02 to 2.61) and lower respiratory infection before 2 years of age were associated with an excess of exercise-induced bronchospasm (OR, 1.71; 95% CI, 1.16 to 2.52). The results suggest that unidentified environmental factors contribute to the excess asthma morbidity in poor children [30]. Environmental exposures may contribute to racial disparities in asthma. Hispanic, African-American, and Asian/Pacific Islander mothers experienced higher mean levels of air pollution and were more than twice as likely to live in the most polluted counties compared with white mothers after controlling for maternal risk factors, region, and educational status [Hispanic mothers: AOR, 4.66; 95% CI, 1.92 to 11.32; African-American mothers: AOR, 2.58; 95% CI, 1.00 to 6.62; Asian/Pacific Islander mothers: AOR, 2.82; 95% CI, 1.07 to 7.39] [31].
These findings may provide some support for the association between income inequality and BHR.
There is a global epidemic of increasing asthma, atopic dermatitis, and other allergic diseases, in developing and developed countries. The epidemic will continue to increase and is due to a lifetime of exposures and influences [32-34]. Further study will be needed to validate the association between being hospitalized in infancy and BHR for reinforcing preventive strategies throughout life, starting in the antenatal and perinatal period.
CONCLUSIONS
This study showed that bronchiolitis diagnosed before 2 years of age and low socioeconomic status interacted on children's bronchial hyperreactivity. Prevention of acute respiratory infection in early childhood, especially in low income families, is important to prevent BHR, a precursor of asthma.
ACKNOWLEDGEMENTS
This study was supported as Eco-R & D by Korea Environmental Industry&Technology Institute.
Notes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/