Evaluation of Maternal Toxicity in Rats Exposed to Multi-Wall Carbon Nanotubes during Pregnancy
Article information
Abstract
Objectives
The present study investigated the potential adverse effects of multi-wall carbon nanotubes (MWCNTs) on pregnant dams and embryonic development following maternal exposure in rats.
Methods
MWCNTs were orally administered to pregnant rats from gestational day (GD) 6 through 19 at dose levels of 0, 8, 40, 200, and 1000 mg/kg/day. During the test period, clinical signs, mortality, body weights, food consumption, serum biochemistry, oxidant-antioxidant status, gross findings, organ weights, and Caesarean section findings were examined.
Results
All animals survived to the end of the study. A decrease in thymus weight was observed in the highest dose group. However, maternal body weight, food consumption, serum biochemical parameters, and oxidant-antioxidant balance in the kidneys were not affected by treatment with MWCNTs. No treatment-related differences in gestational index, embryo-fetal mortality, or fetal and placental weights were observed between treated and control groups.
Conclusions
The results show that 14-day repeated oral dosing of MWCNTs during pregnancy induces minimal maternal toxicity at 1000 mg/kg/day in rats. Under these experimental conditions, the no-observed-adverse-effect level of MWCNTs is considered to be 200 mg/kg/day for dams and 1000 mg/kg/day for embryonic development.
INTRODUCTION
Carbon nanotubes (CNTs) have a structure that looks like a graphite sheet rolled into a cylinder-like shape with a nano-size diameter. Single-wall CNTs are 1-2 nm in diameter with a length of 15 µm or less. Multi-wall carbon nanotubes (MWCNTs) consist of a multi-layered carbon cylinder and have an increased diameter of 10-30 µm. CNTs show distinctive electronic, thermal, and mechanical characteristics according to their structures and sizes. These systems are being widely used in electronic engineering, computers, aerospace, architecture, etc. [1]. However, despite their useful characteristics, because they may have potential adverse effects on humans and the environment, their use has been limited until their safety is guaranteed [2,3]. In order for nanomaterials to be widely used as consumer products, they must be thoroughly investigated to assess potential toxicities using animal models.
CNTs are reported to produce toxic reactions in cells and whole animal experiments. As such, they are presumed to be harmful to human health in the event of exposure to CNTs; however, at this time there is insufficient toxicity information available to verify or dispute this assumption [2]. Humans can be exposed to these potentially toxic substances through inhalation, skin contact, feeding, or as an occupational hazard at high concentrations [1]. It has been suggested that inhaled CNTs and other nanoparticles are likely to evade phagocytosis, penetrate lung tissue, and translocate to other organs causing systemic cell toxicity and injury [4]. Inhaled CNTs, while being removed from respiratory organs and entering the gastrointestinal system through mucociliary synergism, could be transmitted through dirty hands unconsciously or through polluted water and food. Recently it was reported that absorption of CNTs following oral exposure led to oxidative damage of DNA which occurred in a dose-dependent manner affecting liver and lung cells [5]. It has also been determined that CNTs can enter primary or cultured human skin cells, inducing the release of pro-inflammatory cytokines, leading to oxidative damage and decreased cell viability [6].
Recently, Zhu et al. [7] confirmed increased DNA damage and DNA mutation in stomach stem cells of mice exposed to MWCNTs, and Pacurari et al. [8] proved that CNTs induce oxidative damages and activate various routes of DNA damage. Karlsson et al. [9] reported that MWCNTs cause serious DNA damage to A549 type II epithelial cell, and Additionally, intraperitoneally administered MWCNT induced mesothelioma in p53(+/-) heterozygote mice [10]. These results strongly suggest that oxidative stress and DNA damage induced by CNTs might be involved in genotoxicity and carcinogenicity. Many substances that cause oxidative stress and effect DNA cause extreme toxicity in pregnant dams and on embryonic development [11,12], but to date there have been no reports on the potential harmful effects of CNTs on pregnant dams and embryonic development.
This study was intended to determine the safety of nanomaterials that may be hazardous to the human body and the environment due to their increased production and use. Since MWCNTs are widely produced in our country we selected this material to evaluate its potential harmful effects on pregnant dams and embryonic development in rats.
MATERIALS AND METHODS
I. Experiment Animals and Rearing Conditions
Male and nulliparous female Sprague-Dawley rats 10 weeks of age were obtained from a specific-pathogen free colony at Orient Bio (Seoul, Korea) and placed on study following one week of quarantine and acclimatization. For mating, two females were placed into a cage with a male rat overnight. Successful mating was confirmed by the presence of sperm in the vaginal smear, and the following 24 hours was designated as day 0 of gestation (GD 0). The mated females were housed individually in polycarbonate cages, and were provided sterilized tap water and fed commercial rodent chow (Samyang Feed, Wonju, Korea) ad libitum. This experiment was conducted following approval of the Chonnam University Animal Experiment Ethics Committee and was performed in accordance with current regulations for animal experiments.
II. Experiment Substance and Medication
MWCNTs (CM-95, 10-15 nm in diameter, ~20 µm in length) were purchased from Hanhwa Nanotech (Incheon, Korea). These MWCNTs have also been designated as an alternative reference material for the sponsorship program for the safety testing of nanomaterials by the Organization for Economic Co-operation and Development (OECD) Working Party on Manufactured Nanomaterials (WPMN). The purity of MWCNTs is 95%, and they consist of 95% carbon and 5% iron [13]. Based on other reports [10], the test article was suspended in a 1% sodium carboxymethylcellulose (CMC) vehicle. The suspension was subjected to ultrasonication for 3 minutes to obtain a more homogenous and dispersed suspension. The test mixture was prepared daily prior to use, and the 1% CMC solution alone was used as the vehicle control. The test mixture was administered by gavage to pregnant rats from GD 6 through 19 at a dose volume of 20 mL/kg/day. Control rats received an equivalent volume of vehicle alone.
III. Experimental Group Composition and Dosage
According to a previous study [14], no toxic effect was found at a dose of 2500 mg/kg in a single oral dose toxicity study. However, in the case of a single intraperitoneal injection, the median lethal dose was 600 mg/kg, and with 12-day repeated intraperitoneal injections, changes in body weight, feed intake, organ weight, and kidney, spleen, lymph node, thymus, and liver histological changes were observed at 60 mg/kg/day. Slight nephropathy was observed even at 6 mg/kg/day. Based on these results, we orally administered 1000, 100, and 10 mg/kg/day to five females per group for 10 days. Maternal toxicity and developmental toxicity was not observed even in the high-dose group. Therefore, we established the highest dose as 1000 mg/kg/day, which is the limit test dose recommended by OECD test guidelines. Using the common ratio of 5, we added high, medium, and low dose groups of 200, 40, and 8 mg/kg/day, respectively, along with a vehicle control group. A total of 12 confirmed mated females were evaluated per group.
IV. Clinical Signs, Body Weight, and Food Consumption
During the experiment, all pregnant females were examined daily for mortality, morbidity, general appearance, and behavior. Maternal body weights were measured on GDs 0, 6, 9, 12, 15, and 20, and individual food consumption was determined on GDs 0, 6, 9, 12, 15, and 19.
V. Gross Findings and Organ Weights
All pregnant females were euthanized and exsanguinated via the aorta on GD 20. A complete gross postmortem examination was performed. The absolute and relative weights of the lungs, adrenal glands, liver, spleen, kidneys, thymus, heart, and ovaries were recorded.
VI. Serum Biochemical Examination
Blood samples were drawn from the posterior vena cava using a syringe with a 24-gauge needle, and were centrifuged at 2800 rpm for 10 minutes within 1 hour of collection. Using a biochemical automatic analysis device (Dir-chem 4000i, Fujifilm Co., Japan), we measured the concentration of aspartate aminotransferase, alanine aminotransferase, total cholesterol, triglyceride, alkaline phosphatase, blood urea nitrogen, creatinine, glucose, total bilirubin, total protein, and albumin.
VII. Caesarean Section
The ovaries and uterus of each female were removed and examined for the number of corpora lutea and the status of all implantation sites, i.e. live and dead fetuses, early and late resorptions, and total implantations were recorded. Resorption was classified as 'early' only when a resorption site resembling a dark brown blood clot with no embryonic tissue was visible, and 'late' when both placental and embryonic tissues were visible upon postmortem examination.
VIII. Analysis of Kidney Oxidation Damage
We analyzed the extent of lipid peroxidation and oxidative damage on kidney tissue which is suggested to be the main target organ of carbon nanomaterials. After crushing the kidney tissue using a glass-teflon homogenous grinder for a few seconds, the smashed tissue was centrifuged at 4℃, at 800 g for 10 minutes. The protein was quantified according to the method of Lowry et al. [15]. Activity of antioxidant enzymes, glutathione concentration, and degree of lipid peroxidation were also evaluated.
A) Measurement of catalase activity
Catalase was measured in accordance with Aebi's method [16]. After putting 1.0 mL of 30 mM hydrogen peroxide (H2O2) solution into a 2.0 mL sample diluted with 50 mM phosphate buffer solution (pH 7.0), light absorbance change was measured at a wavelength of 240 nm at 20℃. The activity of catalase was measured by calculating the amount of enzyme that decomposed 1 µM of H2O2 in 1 minute and that value was indicated as 1 unit.
B) Measurement of glutathione reductase (GR) activity
Zymogen 200 µL was added to 2.5 mL of 0.1 M phosphate buffer (pH 7.6, 0.5 mM EDTA included), 150 µL of 1 mM glutathione disulfide (GSSG), and 150 µL of 0.1 mM nicotinamide adenine dinucleotide phosphate (NADPH). The solution was mixed and maintained at 25℃ for 2 minutes then the decrease of light absorbance was measured [17]. One unit was defined as the amount of enzyme which oxidized 1 M of NADPH in 1 minute.
C) Measurement of glutathione peroxidase (GPx) activity
The methods of Paglia and Valentine were used to measure GPx [18]. GPx, in the presence of cumene hydroxide, catalyzes oxidation of glutathione, and in the presence of GR and NADPH, is changed to a reduced form. At the same time, NADPH is oxidized to NADP+. The decrease of light absorbance was measured at 340 nm.
D) Measurement of glutathione-S-transferase (GST) activity
GST activity was measured in accordance with the method of Habig et al. [19]. For reaction assistance, 1 mL of 2.5 mM 1-chloro-2,4-dinitrobenzene, 0.5 mL of 1 mM glutathione, and 0.1 M phosphate buffer (pH 6.5) were added. The reaction solution was maintained at 25℃ for 5 minutes, zymogen was added, and change of light absorbance was measured at 340 nm for 2 minutes. One unit was defined as the amount of enzyme that crystallized production of 1 mol of 1-chloro-2,4-dinitrobenzene.
E) Measurement of glutathione (GSH) concentration
Measurement of glutathione concentration was analyzed by the method of Moron et al. [20]. The tissue was mixed with 5 µM EDTA, 0.6 mM of 5,5-dithiol-bis(2-nitrobenzoic acid), 0.2 M NADPH, and 0.1 M sodium phosphate buffer (pH 7.5) which contained GR. After cultivating at room temperature for 2 minutes, light absorbance was measured at 412 nm using a standard curve known to represent GSH concentration.
F) Measurement of lipid peroxidation content
A thiobarbituric acid (TBA) reaction method was used for lipid peroxidation analysis [21]. The tissue was mixed with 0.375% TBA reagent and 15% trichloroacetic acid within 0.25-N hydrochloric acid. The reaction mixture was maintained in boiling water for 30 minutes, and then centrifuged for 5 minutes with 1811 g. The light absorbance of the supernatant was measured as 535 nm. Malondialdehyde (MDA), a product of lipid peroxidation, was calculated using the extinction coefficient 1.56×105/M cm, and the result shown as MDA µM/mg protein.
IX. Statistical Method
Data are presented as means±SD. The unit of statistical measurement is the pregnant female or the litter. GraphPad InStat version 3.0 (GraphPad Software Inc., CA, USA) was used for statistical analysis of control and treatment groups. Statistical significance was examined at 5% and 1%.
RESULTS
During the study period, no abnormal findings were observed in the dams of the control, 8 mg/kg, and 200 mg/kg groups. On the other hand, decreased locomotor activity and depression were identified in one dam of the 40 mg/kg group, and 3 dams of the 1000 mg/kg group (data not shown).
Upon measuring the weight of dams (Table 1) to evaluate treatment-related body weight changes, no statistically significant differences were observed between control and treatment animals. In addition, there were no statistically significant differences in maternal weight gain during pregnancy, gravid uterine weight, and corrected body weights between the groups.
Upon measuring the change in food consumption (data not shown), there was a significant increase on GD 19 in the 40 mg/ kg group compared to the control group, but food consumption in the other groups and on other days was similar to that of the control group.
No gross findings were observed in dams of any group upon evaluation on GD 20.
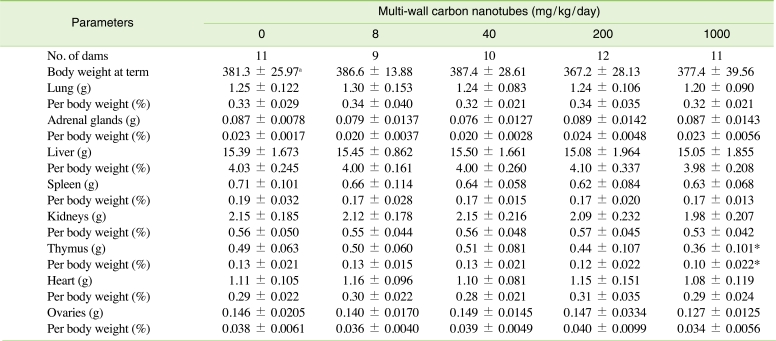
At necropsy (Table 2), there were significant decreases in absolute and relative weights of the thymus in the 1000 mg/kg group, while other organ weights in the treatment groups did not show significant difference compared to the control group.
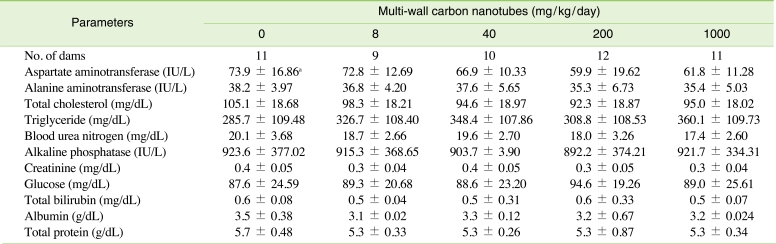
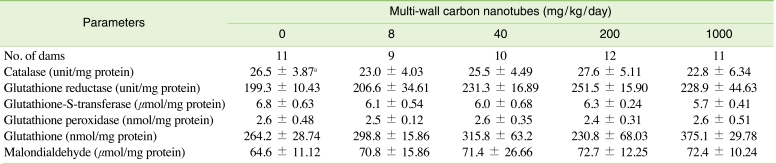
To judge functional abnormalities in the dams, we analyzed serum biochemical values on GD 20 (Table 3). There were no statistically significant differences in the concentrations of aspartate aminotransferase, alanine aminotransferase, total cholesterol, triglyceride, alkaline phosphatase, blood urea nitrogen, creatinine, glucose, total bilirubin, total protein, or albumin between the control and treatment groups. As shown in Table 4, there were also no significant differences observed in catalase, GR, GST, GSH, and MDA levels in the treatment groups compared with those of the control group.
Table 5 summarizes the reproductive and litter findings for the pregnant rats treated with MWCNTs on GDs 6 through 19. The overall pregnancy rate was 83.3-100%, results were similar between each experimental group, and no totally resorbed litters were found in any group. There were no significant differences between the control and treatment groups related to the number of corpora lutea, implantations, pre- and post-implantation loss rates, fetal deaths, litter size, gender ratio of live fetuses, fetal body weights, or placental weights.
DISCUSSION
Physicochemical characteristics of nanomaterials originate from their small size, chemical composition, surface structure, solubility, shape, and condensation. As a result of their unique physicochemical and electrical characteristics, MWCNT usability has greatly increased in various industry sectors, and production is gradually increasing throughout the world [1]. Despite their excellent physicochemical characteristics, the unique features of nanomaterials have aroused concern regarding the effects they may have on humans and the environment. As the interest in the potential adverse effects of nanomaterials increases, scientists continue to evaluate their risks to human health and the environment [3]. According to previous studies, nanomaterials readily travel throughout the body, deposit in target organs, penetrate cell membranes, lodge in mitochondria, and may induce adverse biological effects at cellular, subcellular, and protein levels [2,4]. Therefore, it is thought that the use of nanomaterials should be limited due to possible negative effects on humans and the environment, and confirming their safety through various toxicity experiments should be a top priority [2].
The present study was conducted to investigate potential adverse effects of MWCNTs on pregnant dams and embryonic development in rats orally dosed 0, 8, 40, 200, and 1000 mg/kg/day from GDs 6 through 19. Oxidative damage is not typically measured during standard embryofetal development studies in our laboratory [22]; however, we conducted an additional experiment based on the fact that oxidative stress is thought to play an important role in the manifestation of maternal and embryotoxicity which may be associated with MWCNTs [11]. The results obtained in this study showed that 14-day oral repeated dosing of MWCNTs during pregnancy is minimally maternotoxic, and not embryotoxic in rats.
Results related to CNTs reported by many researchers [3,23] have shown more or less discord in the extent of toxicity manifested in the lungs and other organs [24]. Specifically, it has been reported that CNTs can be harmful to mammalian cells, human T cells [25], HEK293 kidney epithelial cells [26], dermal fibroblast cells [27], and skin epithelial cells [28] in time- and dose-dependent manners. However, it has also been reported that MWCNTs did not induce cytotoxicity or chromosomal aberration in Chinese hamster fibroblast V79 cells [29]. In addition, Di Sotto et al. [30] reported that MWCNTs did not have any mutagenic effects based on reverse mutation tests using germs.
When Wistar rats were exposed to MWCNTs through nasal inhalation for 13 weeks with dosages of 0.4 mg/m3 or more, increases in lung weights, lung lymph nodes, polymorphonuclear neutrophils, harmful collagen of bronchoalveolar cell detergent, and histopathologic changes of the airway occurred. Liang et al. [31] also observed suppressed body weights and increased weights of liver, spleen, and lungs following repeated intraperitoneal injections of MWCNTs at 250 mg/kg for 28 days. Muller et al. [23] reported that after having administered a single tracheal instillation of MWCNTs to rats, inflammatory and fibrotic reactions occurred in the lungs, and the degree of lesions found was dose-dependent. On the contrary, it has been reported that non-purified CNTs did not induce significant signs of lung toxicity 4 weeks after intratracheal administration in guinea pigs [32]. Mitchell et al. [33] reported that even when mice were exposed to MWCNTs through systematic inhalation at a concentration of 0.3-5 mg/m3 for 7 or 14 days, immune function alteration was observed without lung inflammation or tissue damage. Li et al. [34] reported that intratracheal instillation of 0.05 mg of MWCNTs resulted in inflammation of bronchi and severe destruction of alveolar structures, but inhalation exposure of MWCNTs at 32.61 mg/m3 did not cause these pulmonary lesions. Thus, it is considered that differences in lung lesions induced by intratracheal instillation and inhalation of CNTs are caused by the size and distribution of the condensed matter within the lungs. The present study showed that repeated oral administration of MWCNTs caused a decrease in thymus weight in pregnant rats at 1000 mg/kg, but did not cause any harmful effects on embryonic development. The reason why these results do not correspond to results of other researches is considered to be related to factors such as time of exposure, concentration, duration, and physicochemical characteristics of the CNTs used. The difference in absorbance according to route of administration is considered the main cause of the result discrepancies. The results of this study and the existing literature suggest that CNTs do not exert clear and remarkable effects in experimental animals, and products using CNTs are relatively safe for consumer use.
The decreased locomotor activity and depression observed in the 40 mg/kg and 1000 mg/kg groups occurred at a low frequency. These findings are often observed even in normal rats, and as such it is judged that these findings were spontaneous and not related to the MWCNT treatment. Corresponding to the fact that MWCNTs did not have any adverse effects related to clinical findings in pregnant rats, no treatment-related effects were observed in body weight change and weight gain of dams. An increase in food consumption was temporarily observed in the 40 mg/kg group, but this was determined to be an accidental finding unrelated to MWCNT treatment since it did not correspond with body weight change. In addition, according to necropsy results, serum biochemical numerical values, and oxidative damage assessment of the kidneys, no treatment-related effects were found in any group tested. However, the significant decreases in thymus absolute and relative weights observed in the highest dose group were considered to be related to the MWCNT treatment since the decrement was remarkable (about 27 and 23%, respectively) compared to the control group and showed a clear-cut dose-response relationship. Recently, Mitchell et al. [33] reported that mice exposed to inhalation over the whole body at a concentration of 0.3-5 mg/m3 MWCNTs for 7 or 14 days had immune function alterations including T cell antibody-dependent reactions, T cell proliferation, and a decrease of natural killer function, without lung inflammation or tissue damage. Bottini et al. [25] also proved that MWCNTs significantly decrease the viability of cells in a time- and dose-dependent manner through programmed cell death in human T cells. Our research and collected results suggest that administration of MWCNTs in experiment animals could have harmful effects on systemic immune functions and thymus weight, and strongly suggest that the thymus, which is one of the immune organs, is one of the major target tissues of MWCNTs. The exact cause of the decrease in thymus weight was not determined; therefore, it is suggested that additional research be conducted to clarify the mechanism through which CNTs affect the thymus.
CONCLUSION
Fourteen-day repeated oral doses of MWCNTs during pregnancy induce minimal maternal toxicity (i.e., decreased thymus weight) and no embryotoxicity at 1000 mg/kg/day in rats. Under these experimental conditions, the no-observed-adverse-effect level of MWCNTs is considered to be 200 mg/kg/day for pregnant rats, and 1000 mg/kg/day or more for embryonic development. This research is expected to assist in determining the risks of MWCNTs to humans by providing reproductive and developmental toxicity information based on repeated oral exposure of rats during pregnancy.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (2009-0071329). This work was also supported by the Grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Biohousing Research Institute).
Notes
The authors have no conflict of interest to declare on this study.