Toxicity Assessment of Titanium (IV) Oxide Nanoparticles Using Daphnia magna (Water Flea)
Article information
Abstract
Objectives
Titanium dioxide (TiO2), a common nanoparticle widely used in industrial production, is one of nano-sized materials. The purpose of this study was to determine the acute and chronic toxicity of TiO2 using different size and various concentrations on Daphnia magna.
Methods
In the acute toxicity test, four concentrations (0, 0.5, 4, and 8 mM) for TiO2 with 250 or 500 nm and five concentrations (0, 0.25, 0.5, 0.75, and 1 mM) for TiO2 with 21 nm were selected to analyze the toxic effect to three groups of ten daphnia neonates over 96 hours. In addition, to better understand their toxicity, chronic toxicity was examined over 21 days using 0, 1, and 10 mM for each type of TiO2.
Results
Our results showed that all organisms died before the reproduction time at a concentration of 10 mM of TiO2. In addition, the exposure of anatase (21 nm) particles were more toxic to D. magna, comparing with that of anatase (250 nm) and rutile (500 nm) particles.
Conclusions
This study indicated that TiO2 had adverse impacts on the survival, growth and reproduction of D. magna after the 21days exposure. In addition, the number of test organisms that were able to reproduce neonates gradually were reduced as the size of TiO2 tested was decreased.
INTRODUCTION
Nanomaterials, such as nanoparticles, nanotubes, fullerenes, nanowires, and quantum dots, have been developed in numerous research laboratories because they have a much greater surface area to mass ratio than micro-scale material, and they have been adopted in many new technologies [1]. The unique properties and applications of nanoparticles derive from numerous attributes; biomolecules, such as proteins, polynucleic acids, and nanoparticles made of metals and semiconductor materials, can serve as magnetic carriers and can provide fluorescence [2]. The use of nanoparticles for clinical applications presents a risk and has the potential for exposure to a toxic hazard [3].
Acute aquatic methods are a convenient analysis of nanoparticle toxicity, and the method uses various organisms including bacteria Vibrio fischeri, Daphnia magna, and thamnocephalus platyurus [4]. Further, to analyze the in vitro toxicity of nanoparticles and micro-scale materials, the interactions between particles and bacteria and their surface properties were investigated [5].
Titanium dioxide (TiO2) is widely used and is high insoluble and thermally stable. Since it has excellent optical performance and electrical properties, TiO2 has been used for the development solar cell energy [6]. In addition, it is used as a cosmetic to protect the skin from sunlight (e.g. a sunblock) and as a pigment to block water from penetrating a house [7]. Based on these characteristics of TiO2, we investigated toxicity via cellular response by the injection of various nanoparticles and solutions [8] in organisms (e.g. mice, Daphnia magna) [9].
In this study, we conducted an experiment to measure the toxicity of TiO2 nanoparticles via a size- and concentrationdependent manner through in vivo acute and chronic toxicity testing using a D. magna according to international standards. The aim of this study was to analyze both the acute and chronic toxicity using D. magna and to meet these goals, we have used three different types of TiO2 particles, i.e. anatase sized 250 nm and 21 nm, and rutile sized 500 nm.
MATERIALS AND METHODS
I. Daphnia magna Culture
The D. magna used in this study was provided by the Korea Institute of Toxicology (Daejeon, Korea). The D. magna was cultured and treated as described in the US Environmental Protection Agency (US EPA) manual [10]. D. magna cultured and raised in a chamber were retained at 20±1℃ in 2 L beakers containing 1.5 L of hard, reconstituted water (0.008 g L-1 KCl, 0.12 g L-1 CaCO3, 0.12 g L-1 MgSO4, and 0.192 g L-1 NaHCO3 in distilled water filtered through a Minipore Milli-Q apparatus). For performance of the reproduction tests the pH of the medium was adjusted to 8.2±0.2. Cultures were maintained under an 18 hours light: 6 hours dark photoperiod. The medium for the D. magna culture was changed three times per week with fresh HRW(hard reconstituted water) and fed with green algae (Selenastrum capricornutum) and YTC (a mixture of yeast, cerophyll, and Trout chow) purchased from Aquatic Biosystem Inc., (Colorado, US). D. magna culture was maintained in 2-liter glass beakers containing 1.6 L of cultured medium and 30-50 daphnids.
II. Acute Toxicity Test
The acute toxicity test was performed according to the standard protocol for D. magna acute test [10]. Approximately 30 neonates aged less than 24 hours were separated in to three groups and exposed to titanium (iv) oxide (Sigma, US) with different sizes and several concentrations for 96 hours in a static test. All test concentrations were prepared using a volume of 50 mL in triplicate and maintained at 20±1℃ during a 96-hours photoperiod of 16 hours light: 8 hours dark without any feeding. Test beakers were covered with vinyl wrap.
III. Chronic Toxicity Test
The chronic toxicity test was performed according to the standard protocol through D. magna chronic toxicity testing [11]; neonates aged less than 24 hours at the start of the test were exposed to approximately three concentrations of each size of the nanoparticles for a period of 21 days. Neonates were fed two to three times per week, and the media and nanoparticle solution were changed, simultaneously. Survival and offspring were observed and monitored every day. The test beakers were maintained in a chamber at 20±1℃ during a 96 hours photoperiod of 16 hours light: 8 hours dark.
IV. Data Analysis
All of the data were obtained from three independent samples carried out simultaneously for error analysis, and the results were shown along with the standard deviation and the correlation between the mortality under several experimental conditions. The data were analyzed using Sigma Plot (SPSS Inc., Chicago, IL. USA). A p value < 0.05 was considered significant.
RESULTS
The acute toxicity of the three other sizes of titanium (iv) oxide was investigated using a 96-hours toxicity test with 10 neonates of D. magna (less than 24-hours old) in one beaker. The chronic toxicity test of the three other sizes of titanium (iv) oxide was performed for 21 days using 10 neonates with one neonate in each beaker. The experiments were conducted according to EPA guidelines. The pH of these toxicity tests was unchanged during the tests. All tests were performed in triplicate.
I. Acute Toxicity of TiO2 Particles
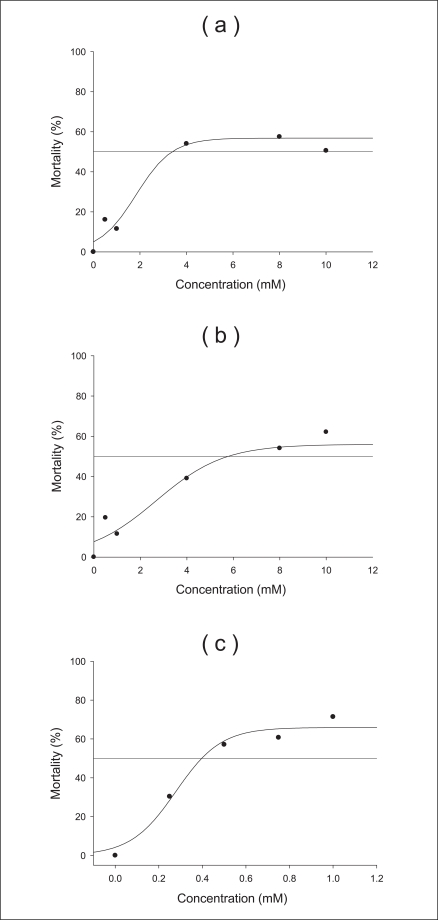
We performed the acute toxicity test for 96 hours with various concentrations and sizes of TiO2 particles. The acute experiment determined the concentration range to be used in the survival experiments. Sodium phosphate buffer (pH 6, 0.1 M) was used as the control, and its mortality was shown in Figure 1. All sizes and concentrations of TiO2 were not acutely toxic over 48 hours. After 96 hours, four neonates treated with anatase (250 nm) and rutile (500 nm) particles at 8 mM survived, but of those treated with anatase (21 nm) at 0.75 mM, four survived. From these results, we determined that the higher concentration led to a higher mortality (see all graphs). Furthermore, the smaller size led to a higher mortality (Figure 1c). Given these results, we found that the regression curves of the acute toxicity data, LC50 and LC20 were determined as the lethal concentrations of a chemical when the percentage of D. magna mortality was 50% and 20%, respectively. In this study, D. magna was exposed to two different LC values (LC20, LC50) of anatase (250 nm) 1.29 and 3.47 mM, respectively, for 96 hours to examine the effects of the anatase form (250 nm) of TiO2 (Figure 2a). Likewise, characteristics of the rutile (500 nm) and anatase (21 nm) varieties were measured. The LC20 and 50 of the rutile (500 nm) and anatase type (21 nm) particles were 1.98, 5.94, 0.19, and 0.42 mM, respectively (Figure 2b, c). Based on the above results, the D. magna toxicity of the three types of TiO2 particles decreased from anatase (21 nm) to anatase (250 nm) to rutile (500 nm) particles.
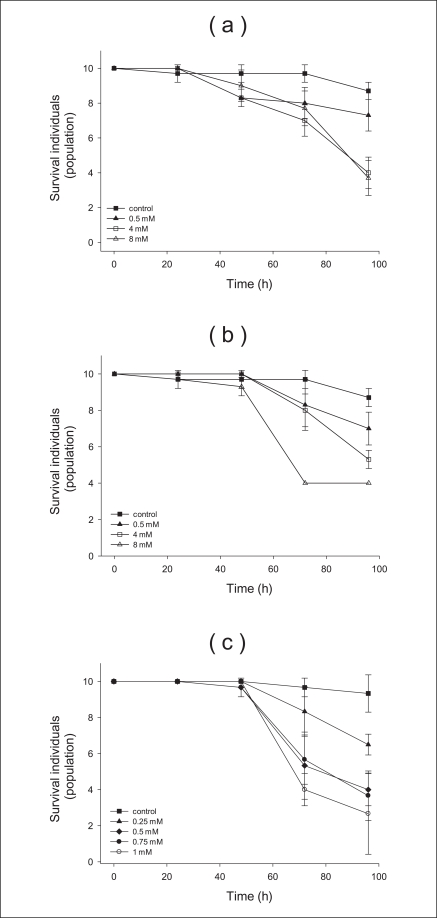
Cumulative parent survival for each of the tested nanoparticles in the 96-hours acute toxicity test: (a) anatase (250 nm), (b) rutile (500 nm), and (c) anatase (21 nm) particles.
II. Chronic Toxicity of TiO2 Particles
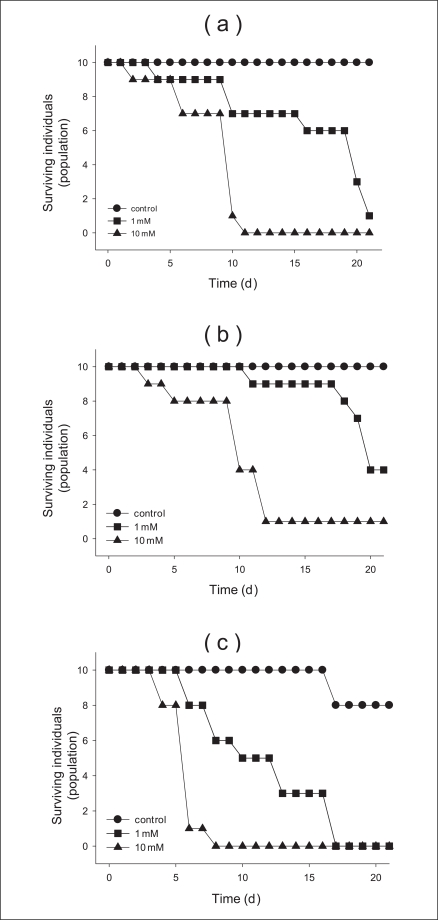
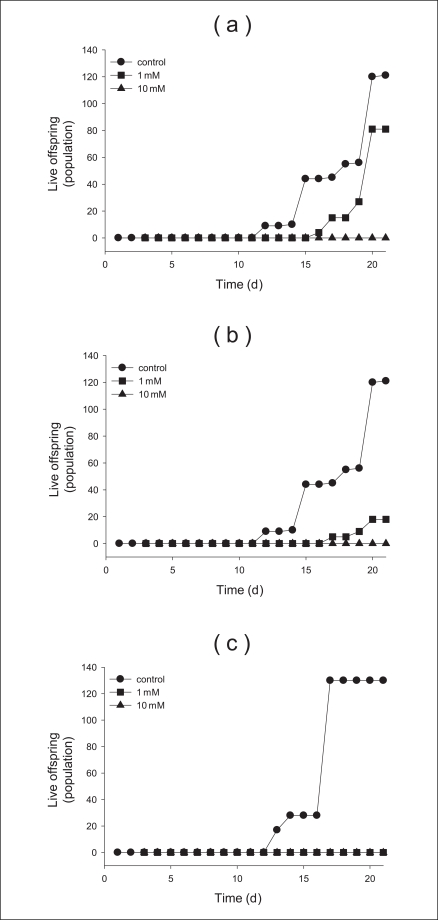
In addition, the chronic toxicity over 21 days using various concentrations and sizes of TiO2 particles determined in this study. Moreover, we used and compared the concentrations (0, 1, 10 mM) in all chronic toxicity tests. We measured the concentration- and size-dependent survival of neonates over 21 days. Survival of anatase (250 nm) and rutle (500 nm) were observed at approximately 10, 40% at 1 mM (Figure 3a, b). In contrast, the survival for 1 mM of anatase (21 nm) particles was not measured because there were killed after the cells were exposed to the solution for 16 days (Figure 3c). From these results, we determined that the toxic response of anatase (250 nm) and rutile (500 nm) progressed slowly, but the response of anatase (21 nm) particles progressed rapidly. There were more than 120 live offspring of the control after 21 days of testing (Figure 4a). That of the anatase (250 nm) and rutile (500 nm) particles with 1 mM was approximately 80 and 20%, respectively. However we did not find any neonates after exposure to anatase (21 nm) particles at 1 mM (Figure 4). Further, we found that no offspring were found at the 10 mM concentration of additional sizes of TiO2 (Figure 4c). After finishing the chronic toxicity tests, we measured the size of neonates living until last day using microscope. Averagely, the size of neonates with 0, 1, and 10 mM anatase (250 nm) materials is 4, 3, and 2 centimeters, respectively. For rutile (500 nm) nanoparticles, the sizes are 4, 2.5, and 2 centimeters, respectively (Figure 5). However, the size of anatase (21 nm) particles could not be measured because all neonates died.
Cumulative live offspring produced per female for each of the tested nanoparitcles after 21 days of exposure: (a) anatase (250 nm), (b) rutile (500 nm), and (c) anatase (21 nm) nanoparticles.
Cumulative parent survival for each of the tested nanoparticles subjected to a chronic toxicity test for 21 days: (a) anatase (250 nm), (b) rutile (500 nm), and (c) anatase (21 nm) nanoparticles.
DISCUSSION
The D. magna showed a very sensitive response dependent upon the size and concentration of TiO2 nanoparticles in both acute and chronic toxicity tests. In particular, the size difference of the nanoparticles demonstrated a greater response than the concentration. As shown in Figure 1, while the toxicity of anatase (250 nm) and rutile (500 nm) particles demonstrated similarities, the smallest size indicated TiO2 induced the greatest toxicity despite the low concentrations of the solution. In a study similar to ours, D. magna was used to confirm that toxicity is influenced by the size of TiO2 nanoparticles [12]. Furthermore, we investigated the same concentration of anatase (250 nm) and rutile (50 nm) particles to compare the toxicity of three types of TiO2. However, no neonates survived the addition of anatase (21 nm) particles (data not shown). The result is likely a rapid absorption of the anatase (21 nm) particles in the neonate because of the small size [13]. Furthermore, oxidative DNA damage occurs in D. magna with TiO2 exposure [14]. We suggest that the neonates have a higher potential for reaching deep into the lungs or damaging internal organs because of the size of TiO2 particles.
CONCLUSIONS
Our innovative approach based on a combination of traditional ecotoxicology methods allowed the clear differentiation of the toxic effects of nonmetal oxide nanoparticles. In addition, this is the first 96-hours evaluation of an acute toxicity test of the size dependence of TiO2 using D. magna. All TiO2 nanoparticles tested by D. magna were sensitive to concentration, and D. magna is widely used in aquatic risk assessment. On the basis of these acute toxicity test results, we conducted a chronic, in vivo toxicity test to confirm dependence on the TiO2 particle size. Acute and chronic effects on D. magna were observed in this investigation.
These responses are due to adaptations, such as changes of state, which happen after exposure to toxicant nano-sized materials. D. magna showed specific response patterns according to toxic effects caused by the size and concentration of TiO2 nanoparticles. The behavior manners of D. magna exposing by different sized TiO2 were considerably different. Therefore, the size effect of TiO2 nanoparticles should be considered in hazard evaluations for the potential to impact aquatic life.
ACKNOWLEDGEMENTS
This work was supported by Mid-career Researcher Program through NRF grant funded by the MEST (No. R01-2008-000-20773-0). The authors are grateful for their support.
Notes
This article is available from: http://e-eht.org/
The authors have no conflict of interest to declare on this study.
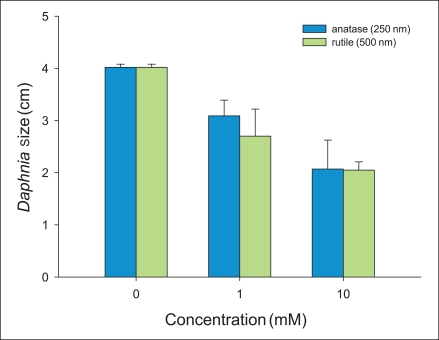
 ) anatase (250 nm) and (
) anatase (250 nm) and ( ) rutile (500 nm) nanoparticles.
) rutile (500 nm) nanoparticles.