Lack of Mutagenicity Potential of Periploca sepium Bge. in Bacterial Reverse Mutation (Ames) Test, Chromosomal Aberration and Micronucleus Test in Mice
Article information
Abstract
Objectives
The root barks of Periploca sepium Bge. (P. sepium) has been used in traditional Chinese medicine for healing wounds and treating rheumatoid arthritis. However, toxicity in high-doses was often diagnosed by the presence of many glycosides. The potential mutagenicity of P. sepium was investigated both in vitro and in vivo.
Methods
This was examined by the bacterial reverse mutation (Ames) test using Escherichia coli WP2uvrA and Salmonella typhimurium strains, such as TA98, TA100, TA1535, and TA1537. Chromosomal aberrations were investigated using Chinese hamster lung cells, and the micronucleus test using mice.
Results
P. sepium did not induce mutagenicity in the bacterial test or chromosomal aberrations in Chinese hamster lung cells, although metabolic activation and micronucleated polychromatic erythrocytes were seen in the mice bone marrow cells.
Conclusions
Considering these results, it is suggested that P. sepium does not have mutagenic potential under the conditions examined in each study.
INTRODUCTION
For thousands of years, Chinese herbal medicines have been used in the treatment of illness by the Chinese and other Asians. The root bark of Periploca sepium Bge. (P. sepium), belonging to the Asclepiadaceae plant family, has a special smell called "HYANGAPI", which means smell cortex. This has been shown to cause dizziness, when humans are exposed for a long time domestically [1]. P. sepium has been used for the treatment of many diseases, such as cardiac palpitation, rheumatoid arthritis, edema of the lower extremities, and shortness of breath [2]. Arthritis, accompanied by aching and a weakness of the lower part of the back and knees is a common symptom [2]. Many chemical components have been found in P. sepium over the years, including sterides, oligosaccharides, pregnane glycosides, cardiac glycosides, coumarins, flavonoids, 2-hydroxy-4-methoxybenzaldehyde and triterpenoides, all of which have pharmacological actions such as anti-inflammatory, cardiac, anti-radiation, antimicrobial, antioxidant and anti-tumor activities [3-7]. The following fractions were identified from P. sepium: steroidal glycoside C21, the perisesaccharides A-E, periplosin, and the periplocosides A, B, C, D, E, F, J, K, L, M and O [8-11]. Periplocoside E inhibited T cell proliferation and modulated the immune system, while periplocoside A prevented concanavalin-induced hepatitis in C57BL/6 mice [12,13]. Periplosin inhibited the growth of lung cancer and had an immunoregulatory effect in tumor-bearing mice [14]. However, it is suggested that there is a safety concern because high dose toxicity was reported in the form of cardiac glycosides [15]. Little is known about the toxicity of P. sepium.
The bacterial reverse mutation (Ames) test is a biological assay that is used to evaluate the mutagenic activity of medical compounds. For the initial screening, the Ames test is used worldwide to determine the mutagenic activity of new medicines as many carcinogenic materials were shown to have a high predictive value in rodents when a positive result is obtained [16,17].
The chromosome aberration test, which used mammalian cells, is a proven in vitro short-term assay to evaluate the genotoxic risks of test materials and guidelines are provided by regulatory offices. Briefly, the method involves culturing cells, exposing them to a test material, harvesting the cells, preparing cells for microscope analysis and then counting the frequency of abnormal structures and chromosome aberrations [18].
Micronuclei were first used to quantify chromosomal damage by Heddle [19] and are now recognized as one of the most successful and reliable assays for genotoxic carcinogens [20].
In this study, the potential genotoxicity of P. sepium was examined using the Ames test, the chromosomal aberration test and the micronucleus test in bone marrow samples.
MATERIALS AND METHODS
I. Chemicals
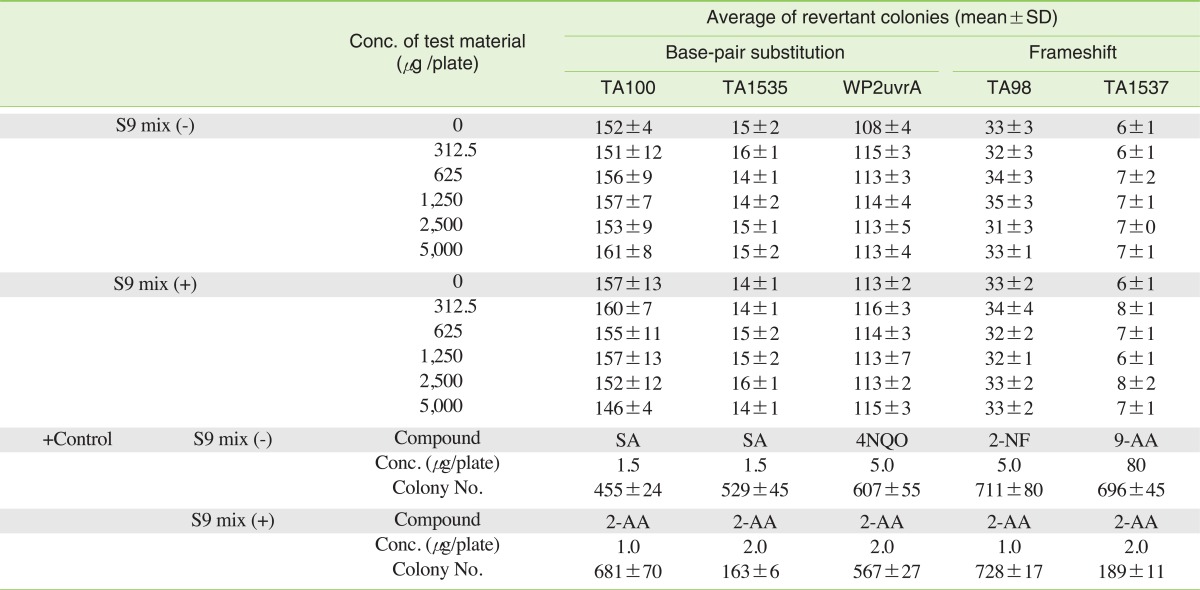
The chemicals and solvents used throughout the experiments were of analytical grade. 2-nitrofluorene and 2-aminoanthracene were purchased from Sigma-Aldrich (St. Louis, MO, USA) and sodium azide, 9-aminoacridine, 4-nitroquinoline-1-oxide, Mitomycin C (MMC) and Benzo[a]pyrene (B[a]P) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dimethylsulfoxide was purchased from Merck & Co., Inc. (Whitehouse Station, NJ, USA).
II. Plant Material
The root bark of P. sepium was purchased from a Korean traditional herbal medicine market. The compound was extracted from the dried root bark of P. sepium using the standard hot water extraction method from the Korean Pharmacopeia. Briefly, P. sepium was added to distilled water (6 to 7 times more than P. sepium) and was boiled for 3 hours at 80-100℃, followed by filtration and concentration under pressure and freeze-drying.
III. Bacterial Strains and Cells and S9
The Salmonella typhimurium Strains TA98, TA100, TA1535 and TA1537 and the Escherichia coli (E. coli) strain WP2uvrA were purchased from Molecular Toxicology Inc. (Boone, NC, USA).
Chinese hamster lung (CHL) cells were purchased from the American Type Culture Collection (Manasass, VA, USA).
A rat liver S9 fraction induced by aroclor in male Sprague-Dawley rats was purchased from Molecular Toxicology Inc. (Boone, NC, USA).
IV. Bacterial Reverse Mutation (Ames) Test
P. sepium was pre-incubated with the test strain and either sterile buffer or the metabolic activation system for 20 minutes or more at 30 to 37℃ prior to mixing with the overly agar and pouring onto the surface of a minimal agar plate. 0.05 mL or 0.1 mL of P. sepium, 0.1 mL of bacteria, and 0.5 mL of S9-mix or sterile buffer, were mixed with 2 mL of overlay agar. Tubes were aerated during pre-incubation using a shaker. For an adequate estimate of variation, triplicate plating was used at each dose level. All plates in a given test were incubated at 37℃ for 48 hours. After the incubation period, the number of reverting colonies per plate was counted.
V. Chromosomal Aberration Test
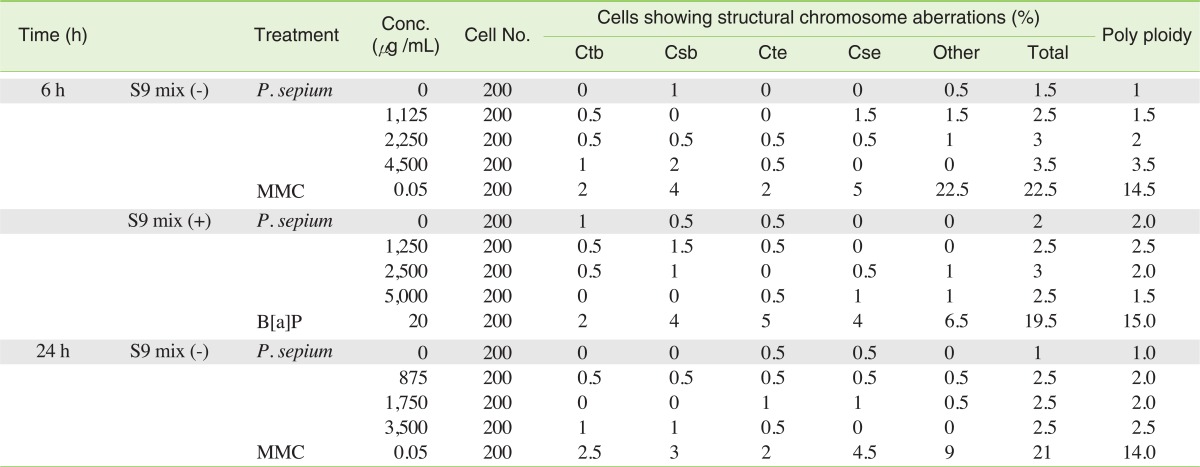
The chromosome aberration test was performed under the following conditions: a short treatment (6 hours) with and without S9, and a continuous treatment for 24 hours to determine the mutagenic potential of P. sepium using CHL cells.
According to the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which was used to determine the highest dose, there was no cytotoxicity even with the application of the S9 metabolic activation system. Therefore, to do the test for the short time treatment (6 hours) with and without the S9 metabolic activation system, concentrations of 1,125, 2,250 and 4,500 µg/mL and 1,250, 2,500 and 5,000 µg/mL, respectively, were chosen for the tests (including the tests on the negative ultrapure water and positive control groups (MMC: 0.05 µg/mL, B[a]P: 20 µg/mL)). For the continuous treatment, 3 doses of treatment groups, such as 875, 1,750, and 3,500 µg/mL were chosen, including the negative (ultrapure water) and positive (MMC: 0.05 µg/mL) control groups.
Two hundred fifty thousand cells (50,000 cells/mL × 5 mL) were seeded in a 60 mm plastic culture dish (LB1), incubated in a culture medium, and then replaced with P. sepium suspended in culture medium. The cells were exposed to P. sepium, MMC or B[a]P for 24 hours (or 6 hours and 24 hours incubation with change of medium). For chromosome preparation, colcemid was added at a final concentration of 0.25 g/mL to the culture medium 2 hours before cell harvesting. Chromosomes were prepared by the air-drying method and stained with 2% Giemsa. The frequency of the cells with various types of structural aberrations including chromatid exchange, chromosome break, chromatid break, chromosome exchange and others (fragmentations, except for pulverization), for each dose in a 200 well-spread metaphase cells (100 metaphase/culture), as well as the cells with numerical aberrations (polyploidy) were recorded.
VI. Micronucleus Test
The micronucleus test was performed on male (ICR) mice, using 5 mice per group that were supplied by the Samtaco Co. Ltd. (Osan, Gyeongi, Korea). The animals were kept in a room with a 12 hours photo period at a temperature of 20 to 25℃ and were provided with animal feed and tap water. The animals were sacrificed at 24 hours, after either the P. sepium had been administered via gavage at doses of 500, 1,000 and 2,000 mg/kg or MMC had been given intraperitoneally at a dose of 2 mg/kg. The treatment concentration of P. sepium and the collection time of the bone marrow cells were chosen on the basis of a preliminary dose-range finding test. The air-dried slides with mice bone marrow micronuclei were stained with 5% Giemsa. The frequencies of micronucleated polychromatic erythrocytes (MNPCE) in 2000 polychromatic erythrocytes (PCEs) per animal and the ratio of PCEs/normochromatic erythrocytes (NCE) in 1000 erythrocytes were determined under a light microscope.
RESULTS
I. Bacterial Reverse Mutation (Ames) Test
Prior to the Ames test, the P. sepium extract was evaluated for the inhibition of growth of the indicator strains used for the tests. The results showed that P. sepium did not inhibit growth with the dosed used (data not shown). The results of the Ames test using the pre-incubation method revealed that the P. sepium extract was non-mutagenic towards S. typhimurium and E. coli (Table 1).
II. Chromosomal Aberration Test
P. sepium did not induce numerical or structural chromosome aberrations even after the application of the S9 metabolic activation system when compared with the negative control group (Table 2).
DISCUSSION
Interest in Chinese herbal medicines is generally due to its traditional use in Asia and the natural origin of the medicines. Although many beneficial biological activities have been scientifically confirmed, caution is called for in the public use of these herbal medicines. Although most herbal products are considered safe if used at the recommended doses, unfavorable effects can still occur [21]. Mutagenicity testing using the Ames test, the chromosomal aberration test and the micronucleus test (the three battery test) is the most frequently used method and is recommended by regulatory agencies for determining genetic risk (Korea Food and Drug Administration, U.S. Food and Drug Administration, Organization for Economic Cooperation and Development). Also, they have a high screening value for carcinogenicity in rodents when a positive result is obtained [22]. In the present study, treatment with P. sepium did not induce mutagenicity in the E. coli strain WP2uvrA or the S. typhimurium strains TA98, TA100, TA1535 and TA1537, with or without metabolic activation. All Salmonella strains are histidine-dependent, and the E. coli strain used in the tests is tryptophan-dependent. Revertant colonies that grow in low levels of tryptophan or histidine were easily identified. Both frameshift and base-pair substitution defects were also found. In this study, P. sepium showed a cytotoxic effect and the doses that were chosen were over 50% greater than live cell concentrations. P. sepium did not induce chromosomal aberrations in either the short-term or the continuous treatment of CHL cells. The chromosomal aberration test was used to find chemicals that induce structural chromosomal aberrations in cultured mammalian cells. Any chemical that has the potential to induce numerical aberrations will show an increase in polyploidy. Chromosome mutations are the cause of many human genetic diseases, and there is potent evidence that chromosome mutations and related mechanisms causing alterations in tumor suppressor genes and oncogenes of somatic cells are concomitant in cancer development in humans. In this study, P. sepium also did not show any induction of the micronucleus in mice bone marrow cells. The formation of micronuclei is an indication of induced chromosome damage. Some toxic metabolites cannot reach the bone marrow cell if their lives are short. Therefore the micronucleus test is a very useful tool for the risk assessment of short life toxic metabolites, which can affect blood lymphocytes. P. sepium is mainly used for the treatment of rheumatoid arthritis, the reinforcement of bones and tendons and the alleviation of aches in the back and knee [2]. Periploside A, a chemical component extracted from P. sepium, possessed comparable bioactivities with lower cytotoxicity in a ConA-induced splenocyte proliferation assay [13]. Also, the periplosides A and E were found to have significant activities against the proliferation of T-lymphocytes in vitro without any obvious cytotoxicity [7,23]. Periplocin, another component, inhibits the viability of lung cancer cell lines, except for National Cancer Institute (NCI)-H292 and NCI-H69 [24]. However, an enhanced survival rate was not observed in athymic nude mice treated with periplocin when they were injected with tumor cells that exhibited the potential toxicity, although obvious pathological changes were not found in many organs. Periplocin inhibits cell growth and down-regulates survival and c-myc expression in colon cancer in vitro and in vivo via beta-catenin/tumor growth factor signaling [25]. However, the cytotoxicity of periplocin was not related to the genotoxicity of CHL cells. Some parts of these chemical components are steroidal, such as pregnane glycoside [7,26]. Some natural steroids, such as androgens, have no genotoxicity and it is suggested that structure similarities have some relationship to the lack of genotoxicity. However, other steroids, such as estradiol, estrone and estriol, showed genotoxicity [27]. In this study, P. sepium was extracted using hot water because it is the same method used in clinical treatments. These results indicate that the clinical treatment of P. sepium may have no mutagenicity to humans.
In conclusion, through the use of the three core test system for genetic damage, it was found that the root bark extracts from P. sepium lack mutagenicity. Future studies are still required for a better knowledge of the chronic toxicity of the extracts of P. sepium.
ACKNOWLEDGEMENTS
This work was supported by the Korea Food and Drug Administration, Republic of Korea, and Grant-in-Aid for National Toxicology Program, 2004.
Notes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/