AbstractAll female Pomacea canaliculata develop a small, male-like copulatory apparatus a few days after birth, which growths slowly until sexual maturity, and even further in older age. Previous studies have found trace elements like mercury (Hg), arsenic (As) and uranium (U) in tap water used for snail culture, and that these elements were accumulated in snail tissues. Here, we test whether the presence of these metals at maximum allowed concentrations (Environmental Protection Agency - EPA) in aquarium water could affect the development of the copulatory apparatus in mature females. Females of different ages were used as controls, grown in reconstituted metal-free water with or without the addition of Hg, As and U, as well as tributyltin (TBT), a compound used as masculinizing agent. Six and seven months old females cultured in tap water showed a longer penis and penile sheath, and a greater overall development of the copulatory apparatus, measured by an index (DI), as compared with same-age females cultured in reconstituted water. Moreover, when females were exposed to Hg, As or U at the maximum contaminant levels for human consumption allowed by EPA regulations, there was no further development of the copulatory apparatus, while there was a clearly positive effect in TBT-exposed females. This study confirms the masculinizing effect of organotin compounds on female copulatory apparatus and discusses the usefulness of the development of these organs as a bioindicator of environmental pollution.
IntroductionGastropods have been proposed as effective monitors of environmental pollution because they can disclose natural, agricultural or industrial emissions of xenobiotics and, in addition, they may be doorways for the entrance of pollutants into the trophic web [1,2].
The freshwater apple snail P. canaliculata (Caenogastropoda, Ampullariidae) has some useful biological features for environmental monitoring. The species is widely distributed in humid tropical and subtropical aquatic ecosystems around the world [3,4] and can be cultured and kept in captivity for laboratory studies. Also, this snail has a high physiological adaptability to different environmental stressors [5–10], which may be facilitated by the presence of genes associated with environmental sensing, complex polysaccharides digestion, and the synthesis of a perivitelline with neurotoxic function involved in the defense against egg predators [11–16].
Likewise, some field and laboratory studies have shown that representatives of the genus Pomacea may be useful for environmental risk assessment of diverse chemical pollutants in nature, i.e., elements (arsenic, As; cadmium, Cd; copper, Cu; mercury, Hg; lead, Pb; uranium, U; zinc, Zn) [10, 17–21], the herbicide glyphosate [22], the insecticides cypermethrin, bifentrin and imidacloprid [23,24]. On the other hand, under controlled laboratory conditions, organotin compounds cause a masculinizing effect on adult (4 months old) females of P. canaliculata [25,26].
Tributyltin (TBT) and Triphenyltin (TPT) induces imposex in some gastropod [27]. More recently, the retinoid X receptor (RXR) has been implicated as a possible molecular mechanism involved [28–31], demonstrating in vitro an increase in RXR transcription levels by TBT and TPT [32].
Unlike the previously mentioned caenogastropods, the genital system of both sexes of P. canaliculata develops from a primordial gonoduct, which can be observed from hatching [33], and from a primordial copulatory apparatus (CApp), which starts to develop between 3–5 days after hatching [33]. In adult males, this CApp reaches full development, while in young females it remains as a rudiment, corresponding to the stage 1 described by Giraud-Billoud and Castro-Vazquez [25]. It is notable, however, that the female CApp would continue its development after sexual maturity is reached, and even in old age. Growth of the female CApp is also promoted by RXR agonists and is associated with a greater expression of the RXR receptor in the CApp [25].
Since apple snails are sensitive accumulators of different elements (As, Ba, Br, Cr, Fe, Hg, Sb, Se, U, and Zn) from water, we evaluated if tap or reconstituted water could represent an important factor in the “normal” development induction of the CApp in adult females. Also, the present work evaluates the development of the CApp in adult females exposed to four elements (Sn, Hg, As, U) administered as tributyltin chloride, mercury dichloride, sodium arsenate, and uranyl acetate which were dissolved in reconstituted water at the highest concentrations allowed by the US Environmental Protection Agency [20].
Materials and MethodsAnimalsAnimals from a cultured strain of P. canaliculata were used. All experiments were carried out with 4-month-old females identified by the presence of a concave operculum [33]. Moreover, the sex was confirmed after the sacrifice by the presence of yellowish ovary associated to the digestive gland and pinkish uterine gland. The stock original and the culture conditions have been reported previously elsewhere [9]. Briefly, room temperature was regulated (23–25 °C) and artificial lighting was provided 14 h per day. The animals were maintained in aquaria containing 6 L of water (i.e., either tap or reconstituted water depending on the experiment; see below). Animals were fed ad-libitum with lettuce from Monday through Friday and this was supplemented with fish food pellets (Peishe Car Shulet®, Argentina) on Thursday and with excess toilet paper on Friday. Food items were provided after water change.
Penis and penial sheath lengths and developmental indexAfter exposure period (Figure 1 and see below), females were immersed in cold water (4 °C for 5 min to minimize nociception), and the shell length (SL) was measured with a caliper. After that, the shell was cracked, the roof of the mantle cavity was removed and the penile length (PL) and penile sheath length (PSL) were measured under a stereoscopic microscope. The CApp developmental index (DI) was also estimated. It includes three mutually exclusive stages [25]. (1) The females with a rudiment penile sheath and a small penile pouch containing a very short penis, (2) females with both distal and medial glands on the penile sheath rudiment, and a penis which could be everted from the penile pouch, and (3) females with a short penile sheath with distal and medial glands and a penile sheath groove, and a coiled penis within the penile pouch.
ExperimentsMasculinization in female snails cultured in reconstituted or tap waterInitially we compare animals cultured in either tap water (TW) or reconstituted water (RW) since tap water showed significant concentrations of several elements in a previous study [20]. In this experiment, the masculinization index in animals cultured from hatching to 4, 5, 6 and 7 months old in either TW or RW was evaluated (Figure 1, green and blue lines, respectively). The aquarium water was changed thrice weekly and then saturated with 1.3 g/L CaCO3 [20]. Reconstituted water was a saline solution (high purity salts) in metal-free American Society for Testing and Materials type I water (ASTM-type I) [10]. Hardness, pH, and alkalinity of reconstituted water (after saturation with CaCO3) were > 286 mg/L of CaCO3, 8.0 and 30.3 mg/L of CO3−2, respectively, while the values in tap water after saturation were > 286 mg/L as CaCO3, 7.8 and 48.2 mg/L as CO3−2, respectively.
Masculinization from apple snails exposed to EPA’s MLC of either Hg, As, or USnails were cultured from hatching to the adulthood in aquaria containing metal-free reconstituted water [20]. Control and experimental female snails were maintained in 6 L aquaria with reconstituted water [10] which was changed weekly. Each age group (4, 5, 6 and 7 months old) cultured in reconstituted water was divided before sacrifice in four experimental subgroups that were exposed for 4 weeks to 6 μg/L of TBT [NOECL reported in 26] as tributyltin chloride (Sigma-Aldrich, T50202) or for six weeks to each of the following compounds, 2 μg/L of Hg as HgCl2 (Sigma-Aldrich, M1136), 10 μg/L of As as Na3AsO4 7H20 (Sigma–Aldrich, S9663), or 30 μg/L of U as UO2CH2COOH (Ted Pella Inc.19481) (Figure 1, orange lines). These elements were selected because of their potential toxicity to humans and the environment and it were used at the maximum contaminant levels (MCLs) for human consumption allowed by EPA regulations. Exposure conditions were semi static and each compound was added after changing the aquarium water. No snails died during the exposure period. Shell length, PL, PSL, and DI were measured as described above.
Statistical analysisFor analysis of the distribution of variables we use a Kolmogorov-Smirnov normality test, and Bartlett’s test for equal variances was used to evaluate homogeneity of variances. Significant differences between groups were evaluated with a Kruskall-Wallis and Dunn’s Tests, while significant differences between two groups were analyzed with a Mann Whitney Test. The p-value stablished was 0.05.
Results and DiscussionMasculinization in female snails cultured in reconstituted or tap waterTemporal changes of reproductive parameters (PL, PSL and DI) in adult females that were cultured in either reconstituted or tap water are shown in Figure 2 (left panels). Shell length from females cultivated in tap water reached higher values than the observed in females cultivated in reconstituted water at 5 (RW=32.2±0.8, TW=37.2±0.8, mean±SEM), 6 (RW=34.4±0.7, TW=39.5±0.7, mean±SEM) and 7 months old (RW=35.4±1.7, TW=38.7±0.5, mean±SEM) (Mann Whitney Test, p <0.05). Adult females cultured in RW did not show significant changes in the masculinization parameters. Moreover, in TW cultured animals, we did not observe any masculinizing change at 4 and 5 months old (mean values: PL=1.2 and 1.7; PSL=1.6 and 1.7; DI =1.40 and 1.53, respectively), but these parameters were significantly increased at 6 and 7 months old in tap water cultured females (mean values: PL=6.3 and 2.3; PSL=2.9 and 2.0; DI=2.53 and 2.46, respectively) (Kruskal-Wallis one-way analysis, Dunn’s test; p< 0.05) (Figure 2, left panels). Furthermore, a principal component analysis was made on the SL, PL, PSL, and DI as variables and the snail age group (cultivated in reconstituted and tap water) as classification criteria. The first two components explain the 96.3% of the variance. Both components clearly separate the PL vector from PSL, DI, SL vectors, although with different variance (PC1=90.8% versus PC2=5.5%). The PSL, DI and SL vectors were more closely associated with older females cultivated in tap water. Moreover, females of 6–7 months old of P. canaliculata cultured under laboratory controlled have showed a developed PSL and an increase in the PL. Here, we show that females cultivated in reconstituted water (4–7 months old) does not develops the CApp with age, compared to females of the same age cultivated in tap water, an effect that could be related to the final size reached at different ages in these groups. Together, these findings indicate that water quality used for snail culture may affect the female “physiological” masculinization that occurs with age.
Masculinization from apple snails exposed to EPA’s MLC of either Hg, As, or U and TBTThe SL in each age group remained approximately constant after exposure to Hg, As, U or TBT (Kruskal-Wallis one-way analysis, Dunn’s test; p> 0.05) (Table 1). An analysis of variance showed that TBT exposed females, regardless of age, significantly increased PL and PSL, reaching the maximum DI (Kruskal–Wallis one-way analysis, Dunn’s test; p< 0.05) (Figure 2, right panels), as it was previously described in females of this species exposed to TBT at NOECL levels [26]. On the other hand, PL and PSL showed no significant changes in females exposed to Hg, As and U among 4 and 7 months old (Kruskall-Wallis one-way analysis, Dunn’s test; p> 0.05) (Figure 2, right panels). A principal component analysis confirmed this result. The components 1 (74.9%) and 2 (24.4%) explain almost all variance and they clearly separate SL from other reproductive parameters. The PL, PSL and DI vectors were more closely associated with TBT exposed females, and they were independent of females’ exposure to Hg, As, U (Figure 2, right panels). The results of this second experiment indicates that females exposed to MCLs-EPA of Hg, As and U, that are accumulated in snail tissues [20], do not have any masculinizing effect in this species, unlike the evident masculinizing effect observed in animals exposed to TBT.
Tin (Sn) (as the organotin compound, TBT) was the only element able to induce any significant change in growth and development (maximum score). This masculinizing effect was independent of the age of the snails and of the composition of the water in which the organotin was dissolved (either tap or reconstituted water), and these organs were similar (but smaller) than the observed in adult males under normal culturing conditions [34]. Likewise, in the ampullariid Marisa cornuarietis, females showed masculinization when are exposed to TBT or TPT [35–37]. However, P. canaliculata was more tolerant than M. cornuarietis at high organotin compounds concentrations. While P. canaliculata tolerates 6 μg/L of TBT [NOECL, 26], M. cornuarietis showed high mortality above 500 ng/L of TBT [37].
AcknowledgementThis work was supported by grants from Agencia Nacional de Promoción Científica, Tecnológica y de Innovación (ANPCYT, FONCyT, PICT 2018-03966 to M. Giraud Billoud; ANPCYT, FONCyT, PICT 2019-03211 to I. Vega), from Universidad Nacional de Villa Mercedes (PROIPO-0222 to M. Giraud Billoud) and from Universidad Nacional de Cuyo (SIIP to M. Giraud Billoud and I. Vega).
NotesCRediT author statement
MGB: Conceptualization, Validation, Formal analysis, Investigation, Resources, Writing - Original draft Preparation, Writing - Review & Editing, Visualization, Funding acquisition; ADCD: Formal analysis, Investigation, Writing - Review & Editing; EMM: Investigation; IAV: Conceptualization, Validation, Formal analysis, Resources, Writing - Original draft Preparation, Writing - Review & Editing, Supervision, Funding acquisition
Conflict of interestThe authors declare that they have no conflicts of interest to report regarding the present study.
References1. Gupta SK, Singh J. Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: a review. IIOAB J 2011;2(1):49-57.
2. Burger J, Gochfeld M. On developing bioindicators for human and ecological health. Environmental monitoring and assessment 2001;66(1):23-46
https://doi.org/10.1023/A:1026476030728
.
3. Hayes K, Burks R, Castro-Vazquez A, Darby P, Heras H, Martín P, et al. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 2015;58: 245-302
https://doi.org/10.4002/040.058.0209
.
4. Cowie RH. Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. Molluscs as crop pests; 2002. 145192
https://doi.org/10.1079/9780851993201.0145
.
5. Giraud-Billoud M, Abud MA, Cueto JA, Vega IA, Castro-Vazquez A. Uric acid deposits and estivation in the invasive apple-snail, Pomacea canaliculata
. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2011;158(4):506-512
https://doi.org/10.1016/j.cbpa.2010.12.012
.
6. Seuffert ME, Burela S, Martín PR. Influence of water temperature on the activity of the freshwater snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) at its southernmost limit (Southern Pampas, Argentina). Journal of Thermal Biology 2010;35(2):77-84
https://doi.org/10.1016/j.jtherbio.2009.11.003
.
7. Yusa Y, Wada T, Takahashi S. Effects of dormant duration, body size, self-burial and water condition on the long-term survival of the apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae). Applied Entomology and Zoology 2006;41(4):627-632
https://doi.org/10.1303/aez.2006.627
.
8. Giraud-Billoud M, Castro-Vazquez A, Campoy-Diaz AD, Giuffrida PM, Vega IA. Tolerance to hypometabolism and arousal induced by hibernation in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2018;224: 129-137
https://doi.org/10.1016/j.cbpb.2017.12.015
.
9. Giraud-Billoud M, Vega IA, Tosi MER, Abud MA, Calderón ML, Castro-Vazquez A. Antioxidant and molecular chaperone defences during estivation and arousal in the South American apple snail Pomacea canaliculata
. Journal of Experimental Biology 2013;216(4):614-622
https://doi.org/10.1242/jeb.075655
.
10. Campoy-Diaz AD, Arribére MA, Guevara SR, Vega IA. Bioindication of mercury, arsenic and uranium in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): bioconcentration and depuration in tissues and symbiotic corpuscles. Chemosphere 2018;196: 196-205
https://doi.org/10.1016/j.chemosphere.2017.12.145
.
11. Giglio ML, Ituarte S, Milesi V, Dreon MS, Brola TR, Caramelo J, et al. Exaptation of two ancient immune proteins into a new dimeric pore-forming toxin in snails. Journal of Structural Biology 2020;211(2):107531
https://doi.org/10.1016/j.jsb.2020.107531
.
12. Dreon MS, Frassa MV, Ceolín M, Ituarte S, Qiu JW, Sun J, et al. Novel animal defenses against predation: a snail egg neurotoxin combining lectin and pore-forming chains that resembles plant defense and bacteria attack toxins. PLoS One 2013;8(5):e63782
https://doi.org/10.1371/journal.pone.0063782
.
13. Frassa MV, Ceolín M, Dreon MS, Heras H. Structure and stability of the neurotoxin PV2 from the eggs of the apple snail Pomacea canaliculata. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2010;1804(7):1492-1499
https://doi.org/10.1016/j.bbapap.2010.02.013
.
14. Dreon MS, Ituarte S, Heras H. The role of the proteinase inhibitor ovorubin in apple snail eggs resembles plant embryo defense against predation. PLoS One 2010;5(12):e15059
https://doi.org/10.1371/journal.pone.0015059
.
15. Sun J, Mu H, Ip JC, Li R, Xu T, Accorsi A, et al. Signatures of divergence, invasiveness, and terrestrialization revealed by four apple snail genomes. Molecular Biology and Evolution 2019;36(7):1507-1520
https://doi.org/10.1093/molbev/msz084
.
16. Escobar-Correas S, Mendoza-Porras O, Dellagnola FA, Colgrave ML, Vega IA. Integrative proteomic analysis of digestive tract glycosidases from the invasive golden apple snail, Pomacea canaliculata
. Journal of proteome research 2019;18(9):3342-3352
https://doi.org/10.1021/acs.jproteome.9b00282
.
17. Callil CT, Junk WJ. Aquatic Gastropods as Mercury Indicatorsin the Pantanal of Poconé Region (Mato Grosso, Brasil). Water, Air, and Soil Pollution 2001;125(1):319-330
https://doi.org/10.1023/A:1005230716898
.
18. Hoang TC, Rogevich EC, Rand GM, Gardinali PR, Frakes RA, Bargar TA. Copper desorption in flooded agricultural soils and toxicity to the Florida apple snail (Pomacea paludosa): Implications in Everglades restoration. Environmental Pollution 2008;154(2):338-347
https://doi.org/10.1016/j.envpol.2007.09.024
.
19. Peña SC, Pocsidio GN. Influence of copper on the feeding rate, growth and reproduction of the golden apple snail, Pomacea canaliculata Lamarck. Bulletin of Environmental Contamination and Toxicology 2007;79(6):606-608
https://doi.org/10.1007/s00128-007-9312-6
.
20. Vega IA, Arribére MA, Almonacid AV, Ribeiro Guevara S, Castro-Vazquez A. Apple snails and their endosymbionts bioconcentrate heavy metals and uranium from contaminated drinking water. Environmental Science and Pollution Research 2012;19(8):3307-3316
https://doi.org/10.1007/s11356-012-0848-6
.
21. Campoy-Diaz AD, Escobar-Correas S, Canizo BV, Wuilloud RG, Vega IA. A freshwater symbiosis as sensitive bioindicator of cadmium. Environmental Science and Pollution Research 2020;27(3):2580-2587
https://doi.org/10.1007/s11356-019-07082-x
.
22. Xu Y, Li AJ, Li K, Qin J, Li H. Effects of glyphosate-based herbicides on survival, development and growth of invasive snail (Pomacea canaliculata). Aquatic Toxicology 2017;193: 136-143
https://doi.org/10.1016/j.aquatox.2017.10.011
.
23. Arrighetti F, Ambrosio E, Astiz M, Capítulo AR, Lavarías S. Differential response between histological and biochemical biomarkers in the apple snail Pomacea canaliculata (Gasteropoda: Amullariidae) exposed to cypermethrin. Aquatic Toxicology 2018;194: 140-151
https://doi.org/10.1016/j.aquatox.2017.11.014
.
24. Attademo AM, Tamburi NE, Peltzer P, Lajmanovich RC, Martinuzzi CS. Metabolic stress and shell thinning in Pomacea canaliculata (Caenogastropoda, Ampullaridae) in rice agroecosystems of Argentina. 2018.
25. Giraud-Billoud M, Castro-Vazquez A. Aging and retinoid X receptor agonists on masculinization of female Pomacea canaliculata, with a critical appraisal of imposex evaluation in the Ampullariidae. Ecotoxicology and environmental safety 2019;169: 573-582
https://doi.org/10.1016/j.ecoenv.2018.10.096
.
26. Giraud-Billoud M, Vega IA, Wuilloud RG, Clément ME, Castro-Vazquez A. Imposex and novel mechanisms of reproductive failure induced by tributyltin (TBT) in the freshwater snail Pomacea canaliculata
. Environmental toxicology and chemistry 2013;32(10):2365-2371
https://doi.org/10.1002/etc.2310
.
27. Titley-O’Neal CP, Munkittrick KR, MacDonald BA. The effects of organotin on female gastropods. Journal of Environmental Monitoring 2011;13(9):2360-2388
https://doi.org/10.1039/C1EM10011D
.
28. Horiguchi T. Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanism of actions of tributyltin and triphenyltin for development of imposex. Environ Sci 2006;13(2):77-87.
29. Lima D, Reis-Henriques MA, Silva R, Santos AI, Castro LFC, Santos MM. Tributyltin-induced imposex in marine gastropods involves tissue-specific modulation of the retinoid X receptor. Aquatic toxicology 2011;101(1):221-227
https://doi.org/10.1016/j.aquatox.2010.09.022
.
30. Nishikawa JI. Imposex in marine gastropods may be caused by binding of organotins to retinoid X receptor. Marine Biology 2006;149(1):117-124
https://doi.org/10.1007/s00227-005-0210-3
.
31. Nishikawa JI, Mamiya S, Kanayama T, Nishikawa T, Shiraishi F, Horiguchi T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environmental science & technology 2004;38(23):6271-6276
https://doi.org/10.1021/es049593u
.
32. Urushitani H, Katsu Y, Kagechika H, Sousa AC, Barroso CM, Ohta Y, et al. Characterization and comparison of transcriptional activities of the retinoid X receptors by various organotin compounds in three prosobranch gastropods; Thais clavigera, Nucella lapillus and Babylonia japonica. Aquatic Toxicology 2018;199: 103-115
https://doi.org/10.1016/j.aquatox.2018.03.029
.
33. Gamarra-Luques C, Giraud-Billoud M, Castro-Vazquez A. Reproductive organogenesis in the apple snail Pomacea canaliculata (Lamarck, 1822), with reference to the effects of xenobiotics. Journal of Molluscan Studies 2013;79(2):147-162
https://doi.org/10.1093/mollus/eyt011
.
34. Giraud-Billoud M, Gamarra-Luques C, Castro-Vazquez A. Functional anatomy of male copulatory organs of Pomacea canaliculata (Caenogastropoda, Ampullariidae). Zoomorphology 2013;132(2):129-143
https://doi.org/10.1007/s00435-012-0183-y
.
35. Schulte-Oehlmann U, Tillmann M, Markert B, Oehlmann J, Watermann B, Scherf S. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part II: Triphenyltin as a xeno-androgen. Ecotoxicology 2000;9(6):399-412
https://doi.org/10.1023/A:1008924602089
.
36. Duft M, Schulte-Oehlmann U, Tillmann M, Weltje L, Oehlmann J. Biological impact of organotin compounds on mollusks in marine and freshwater ecosystems. Coastal Marine Sciences 2005;29(2):95-110.
37. Janer G, Lyssimachou A, Bachmann J, Oehlmann J, Schulte-Oehlmann U, Porte C. Sexual dimorphism in esterified steroid levels in the gastropod Marisa cornuarietis: the effect of xenoandrogenic compounds. Steroids 2006;71(6):435-444
https://doi.org/10.1016/j.steroids.2006.01.012
.
Figure 1Experimental designs. First experiment: copulatory apparatus (CApp) growth and development in female snails cultivated in a reconstituted or tap water (green and blue lines, respectively). Second experiment: snails cultured from hatching to adulthood in reconstituted water and then exposed to organotin (TBT; four weeks) or different elements (Hg, As, and U; six weeks) (orange lines). At the end of the experiments (4, 5, 6 and 7 months old), the snails were sacrificed and the shell length and different reproductive parameters were measured. 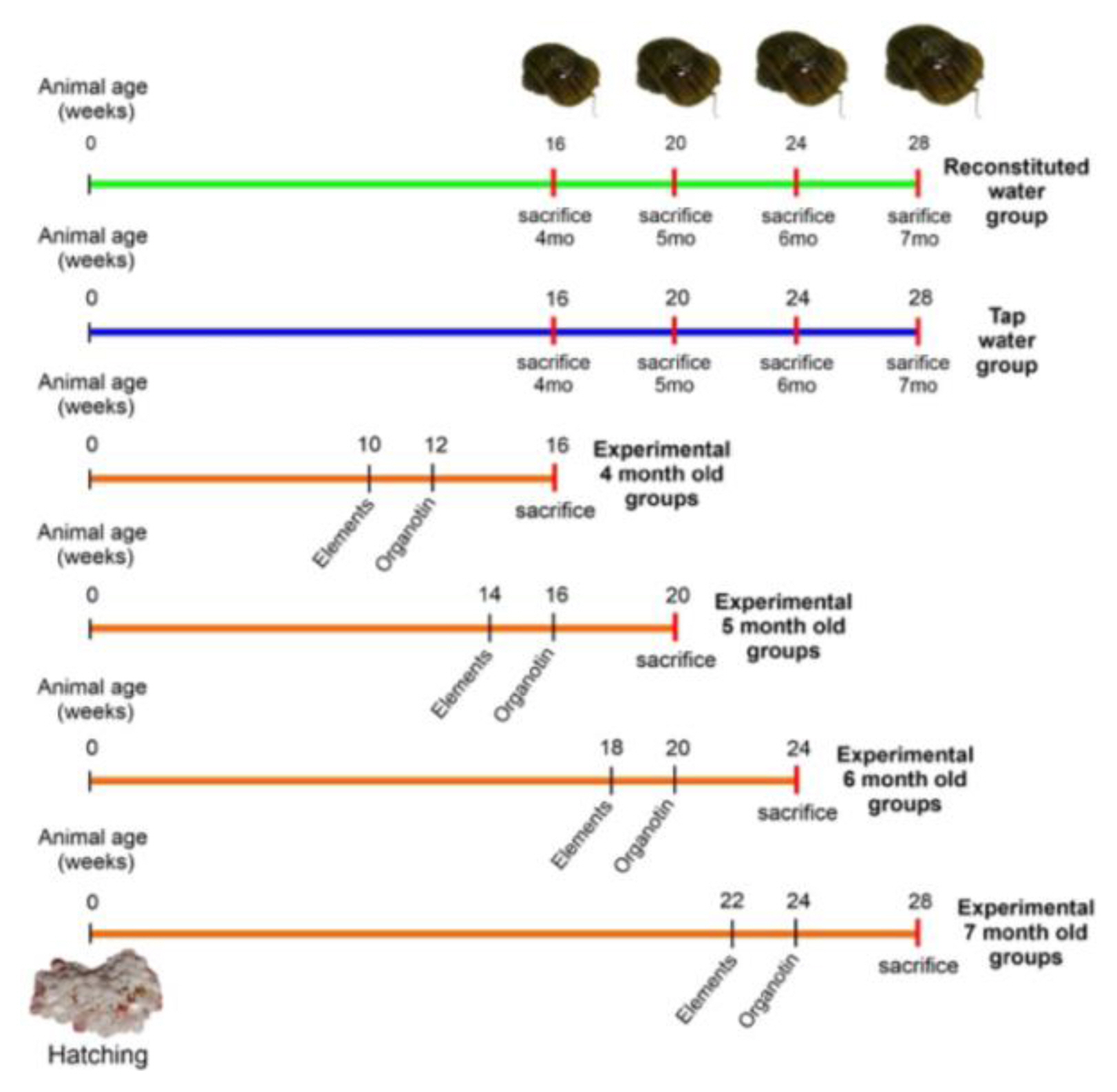
Figure 2Left panels show reproductive parameters (penile length -PL-, penile sheath length -PSL-, developmental index -DI-) of females that were cultured from hatching to 4, 5, 6 and 7 months old either in tap (TW) or reconstituted water (RW). Equal letters indicate significant differences among groups (p< 0.05, Kruskall-Wallis, Dunn’s Tests) of females at different ages under the same culture conditions; asterisks indicate differences between equal month-old females that were cultured in TW or in RW (p< 0.05, Mann Whitney Test). Principal components (PC) analysis using shell length (SL), PL, PSL, and DI (yellow circles) as variables and the age and both water qualities as a criterion of classification (blue circles). Right panels show females cultured in RW from hatching to 4, 5, 6 and 7 months that were exposed to TBT (NOECL, 6 ug/L) for 4 months, and Hg (2 μg/L), As (10 μg/L) or U (30 μg/L) for 6 months. One asterisk indicates differences between TBT and Hg, As, and U, and two asterisks indicate differences between Hg or U and TBT (p< 0.05, Kruskall-Wallis, Dunn’s Tests). PC analysis using the SL, PL, PSL and DI (yellow circles) as variables and the age and the treatment as a criterion of classification (blue circles). 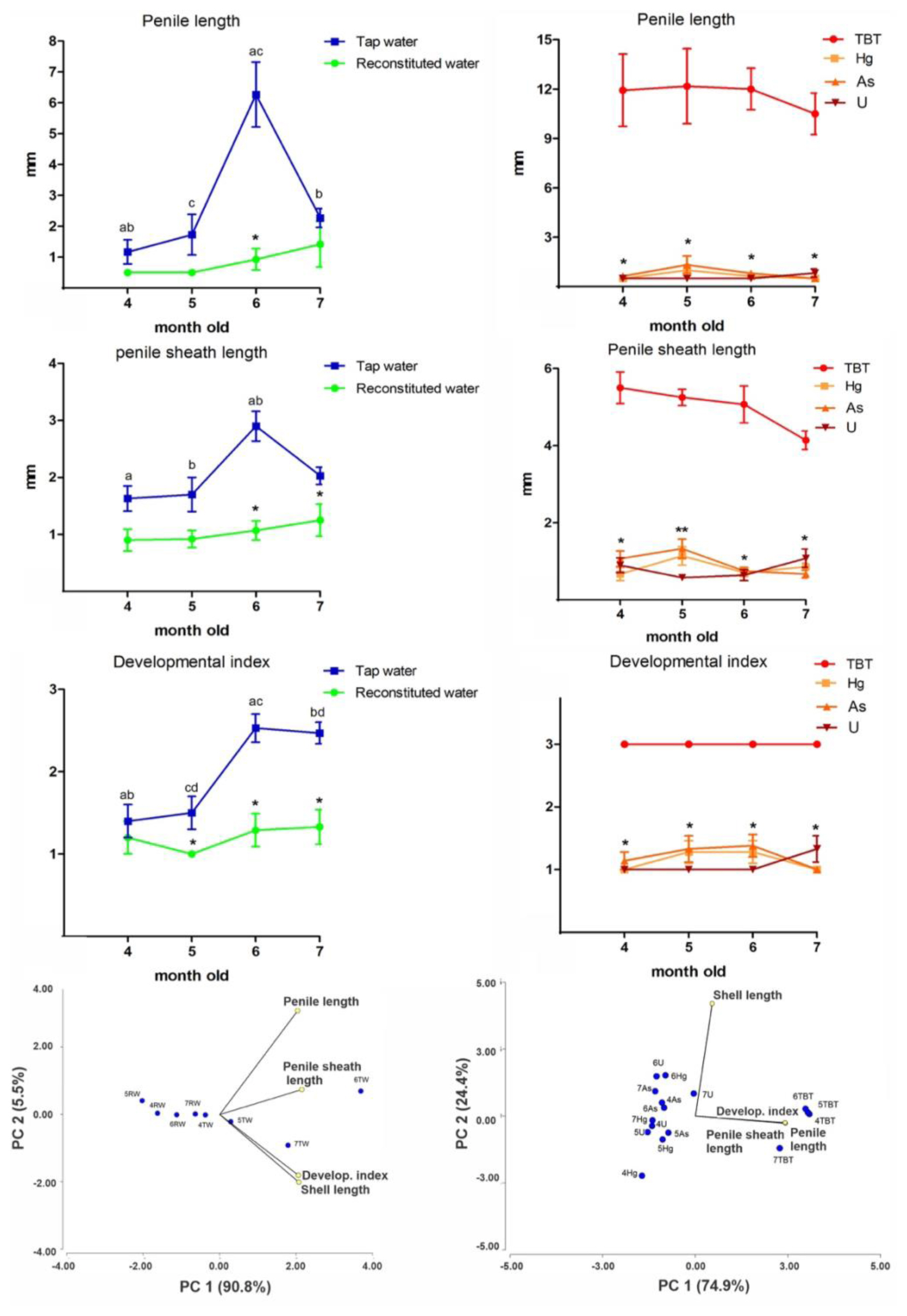
Table 1Shell length of P. canaliculata females cultured in reconstituted water and then exposed to mercury (Hg), arsenic (As), uranium (U), or tributyltin (TBT).
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||