AbstractThe types and mechanisms of atrazine-metolachlor toxicity, an herbicide composed of atrazine (ATR) and metolachlor (MET), need to be further investigated. This study evaluated the toxic actions of ATR-MET by in vivo and in silico methods. Here, varying doses of ATR-MET were orally administered to rats once daily for twenty-one days using normal saline as control. Molecular docking was used to characterize the binding of ATR and MET with androgen receptor (AR) to predict their potential endocrine-disrupting effects, using testosterone as benchmark. ATR-MET-induced-testicular toxicity (reduced sperm motility, count, and daily sperm production and increased live/dead ratio) was accompanied with testicular oxidative stress (diminished level of reduced glutathione, activities of glutathione-S-transferase, superoxide dismutase and catalase and increased level of malondialdehyde). Furthermore, ATR-MET induced cardiovascular toxicity (increased levels of plasma total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides) with concomitant induction of renal toxicity (increased plasma creatinine and urea levels), and hepatotoxicity (increased plasma bilirubin, alkaline phosphatase, acid phosphatase, alanine aminotransferase and aspartate aminotransferase). Binding energy and amino acid interactions from in silico study revealed that MET possessed endocrine-disrupting capacity. In conclusion, exposure to atrazine-metolachlor could promote cardiovascular, renal, hepatic, as well as reproductive impairment in experimental male albino rats.
IntroductionAtrazine (ATR)-metolachlor (MET) is an herbicide composed of a mixture of ATR emulsion and MET used to control a broad array of weeds mainly in cultivated farmlands. Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine, Figure 1) is a selective herbicide that is effective against broadleaf weeds while metolachlor (MET) controls grasses; but used together as a mixed herbicide, ATR-MET provide efficacious management of weeds appearing as broadleaves and grasses [1,2]. Thus, instead of using two different herbicides, one for broadleaf and the other for grasses, ATR-MET as a combination herbicide is used; this is effective, saves time and resources. ATR, on its own is an herbicide in widespread application globally, and the common formulations with MET include 200 g/L of ATR+200 g/MET suspension-emulsion, 270 g/L ATR+150 g/L MET suspension-emulsion, 370 g/L ATR+290 g/L MET suspension concentrate [2]. It is considered an environmental contaminant [3] because it has been detected in groundwater, food, drinking water, the atmosphere and even in human body; with atrazine detected in 20% of indoor air evaluated at concentration of about 200–300 ng/m for atrazine, using an air sampler [4–6]. In spite of its wide occurrence, the harmful effects of ATR exposure on humans are still not completely clear, although epidemiological studies have shown that exposure to ATR may adversely affect reproductive and developmental progression [7], while various animal studies have shown the adverse effect of ATR on the endocrine, reproductive and the development of the central nervous systems [4]. For instance, it has been shown that ATR reduces the number and motility of sperms in rats and in man [8–10], impedes reproductive maturation in both male and female rats [3, 11], decreases prostate and seminal vesicle weights in rats [3,11]. The hepatotoxicity of atrazine has also been reported by some studies. For example, in Wistar strain of albino rats, atrazine was shown to cause hepatotoxicity which was revealed by increased manifestation of hepatic disease indices including dose dependent reduction in serum glucose concentration, increase in total serum lipids, and the activities of serum alanine amino transferase (ALT), alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT) [12,13].
Metolachlor (2-chloro-N-(2-ethyl-6-methylphenyl)-N-(2-metoxy-1-methylethyl) acetamide, Figure 2) on the other hand is a discriminatory herbicide from chloroacetamide group which hinder seed germination by inhibiting mitosis and cell division [14], as well as preventing the synthesis of chlorophyll, fatty acids, lipids and proteins [15]. Like atrazine, metolachlor has also been found to linger on agricultural soil, in surface waters as well as groundwater [16–18]. Studies have also shown that metolachlor could adversely affect the functions of the male reproductive system by various mechanisms including modulation of testosterone production via increase in the activity of aromatase enzyme in a human cell line, as well as via other mechanisms [15,17–19].
As stated above, ATR-MET is ATR in combination with MET, however, little is known about the toxicity of this formulation. Investigation of alterations in cardiovascular, hepatic and renal disease indices, antioxidant parameters, male-related organ toxicity and related enzymes, and increases of lipid peroxidation levels, offer rapid means to assess toxic effect and mechanism of action of chemicals, including herbicides. These biochemical end points have been used to evaluate potential adverse effect of chemicals to explain their adverse outcomes. Accordingly, this study was undertaken to evaluate the effect of varying combinations of ATR and MET on selected organ disease indices, including cardiovascular, hepatic, renal and the male reproductive system. To achieve this, the study used cardiovascular, hepatic, renal and testicular toxicity and antioxidant indices in male rats to investigate the toxic effects of the administration of varying concentration of ATR-MET. The study also employed molecular docking to characterize the structural binding of atrazine and metolachlor with androgen receptor (AR) to predict their potential endocrine-disrupting effects in AR signalling.
Materials and MethodsAnimals and reagentsTwenty-four albino male rats (Ratus novergicus) with an average weight of 140 g used for this study were purchased from the Animal Holding Unit of the Department of Biochemistry, University of Ibadan, Nigeria. ATR-MET is a product of First Chemical Co Ltd, Changxing, Zhejiang China. GSH, 1-chloro-2, 4-dinitrobenzene (CDNB), 5′, 5′-dithio-bis-2-nitrobenzoic acid (DTNB), thiobarbituric acid (TBA), epinephrine and hydrogen peroxide were purchased from Sigma Chemical Company (London, UK). ALT, AST, ALP, GGT, urea, creatinine, bilirubin, total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides kits were obtained from Randox Laboratories Ltd, Antrim UK. All other chemicals and reagent were of analytical grade and were obtained from British Drug House, Poole, UK.
Animal handling and treatmentThe experimental animals were handled and used in accordance with the international guide for the care and use of laboratory animals [20]. They were kept in normal laboratory conditions under natural light–dark cycle with access to food and water ad libitum all through the time of the experiment. The animals were randomly assigned into four groups (of 6 rats each), which were designated A (control), B, C and D. The control rats in group A were administered physiological saline orally for 21 days while rats in groups B, C and D were orally administered ATR-MET 1 (67.5 mg ATR+37.5 mg MET kg/BW), ATR-MET 2 (135 mg ATR+75 mg MET kg/BW) and ATR-MET 3 (270 mg ATR kg/BW+150mg MET kg/BW) daily for 21 days respectively. These doses were set independently based on earlier studies [21,22]. The drugs were administered as homogenous aqueous suspensions in normal saline. All the animals were sacrificed 24 hours after the twenty-first day of drug administration.
Collections of plasma and tissue samplesThe rats were sacrificed by cervical dislocation. Blood samples were collected by ocular puncture into heparinized tubes. The blood samples were centrifuged for 10 min at 4000 g (Cencom bench centrifuge) to obtain the plasma which was thereafter kept frozen for the estimation of cardiovascular indices (lipid profile), renal and liver function test, as well as other marker enzymes. The testes were removed from the animals, rinsed in ice-cold 1.15% KCl, blotted and weighed, and thereafter used to prepare sub-cellular fraction.
Preparation of sub-cellular fractionThe testes were weighed, macerated and homogenized in 4 volumes of ice-cold 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuge at 12,500 g for 15 mins using an Eppendorf refrigerated centrifuge. The supernatant, termed the post mitochondria fractions (PMF), was obtained and stored frozen for subsequent analysis.
Analysis of sperm parametersTesticular sperm number, progressive sperm motility assay, and volume.
Testicular sperm was obtained by mincing the cauda epididymis and the testis in normal saline and filtering through a nylon mesh. The spermatozoa were counted using the Neubauer haemocytometer following the methodology as described by Pant and Srivastava [23]. The motility of epididymal sperm was evaluated visually at 400xmagnification within 2–4 min of isolation from the cauda. Motility estimations were performed from the entire field in each sample. The mean was used as the final motility score and data were expressed as percentages [24].
Morphological and live-dead examination of spermatozoaA portion of the sperm suspension placed on a glass slide was smeared out with another slide, fixed in 95% ethanol, and stained with 1% eosin and 5% nigrosine for morphological and viability observation. At least 100 sperms from each rat were examined for abnormalities in different regions of spermatozoa according to the method described by [25].
Daily Sperm Production (DSP) rateThe testis was weighed, decapsulated and homogenized in ice-cold 0.9% sodium chloride. The homogenate was filtered through a nylon mesh to remove connective tissue, and the filtrate was used to count the number of homogenization-resistant spermatids/sperm in each sample in duplicate using a haemocytometer. DSP was calculated by dividing the total number of spermatids/sperm per gram testis by 6.1 days, the duration of step 19 spermatids in the seminiferous tubules [26].
Assay of biochemical parametersThe protein content of the samples was determined according to the Biuret method, using bovine serum albumin as standard [27]. Briefly, the biuret method depends on the principle that when a solution of protein is treated with Cu2+ in moderately alkaline medium, a purple colored chelate is formed between Cu2+ and the peptide bonds of the protein with maximum absorbance at 540 nm. The intensity of the purple color is proportional to the amount of protein present. Activities of ACP according to the method of Tietz et al. [28], ALP by the method of Wright et al. [29], AST and ALT following the principle described by Reitman and Frankel [30], the levels of creatinine and urea by the method described by Tietz et al. [28] were determined by using Agape Diagnostics, Switzerland GmbH assay kits following the manufacturer’s instruction. Catalase activity was determined according to the method of Sinha, 1972 [31]. Briefly, a reaction mixture of 2 mL of hydrogen peroxide (800 μ moles), 2.5 mL of 0.01 M phosphate buffer (pH 7.0) and 0.5 mL of diluted sample (1:50) was rapidly constituted at 25 °C, 1 mL of reaction mixture was withdrawn and promptly added to 2 mL dichromate/acetic acid solution at intervals of 1 min to measure the residual H2O2 in the solution. The chromic acetate generated was quantified at 570 nm and the residual H2O2 estimated extrapolating from the standard curve for hydrogen peroxide. The activity of catalase was expressed as micromole of H2O2 used up/min/mg protein.
The activity of SOD was estimated by the method of Misra and Fridovich [32]. Briefly, 1 mL of the sample was diluted in 9 mL of distilled water to get a 1:10 dilution; 0.2 mL of the diluted enzyme preparation was added to 2.5 mL of 0.05 M carbonate buffer (pH 10.2) and allowed to equilibrate in the spectrophotometer. The reaction was started by the addition of 0.3 mL of newly prepared 0.3 mM epinephrine to the mixture which was quickly mixed by inversion, the reference cuvette contained 0.2 mL of distilled water, 2.5 mL of carbonate buffer and 0.3 mL of epinephrine. The increase in absorbance at 480 nm was monitored every 30 s for 150 s. The activity of SOD in the sample was expressed as follows:
One Unit of SOD activity is taken as the amount of SOD necessary to cause 50% inhibition of the oxidation of adrenaline to adrenochrome over an interval of 60s.
SOD activity (Unit/mg protein)=(df=dilution factor). The level of GSH was determined by the method described by Jollow et al. [33]. Briefly, the reaction mixture contained 1.8 mL of distilled water, 0.2 mL of sample and 3 mL of 4% sulphosalicylic acid. The reaction mixture was allowed to stand for 5 minutes, filtered, 1 mL of the filtrate was added to 4 mL of 0.1 M phosphate buffer and finally, 0.5 mL of Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid), DTNB), (0.04% in 0.1 M phosphate buffer, pH 7.4) was added. A blank was prepared with 4 mL of the 0.1 M phosphate buffer, 0.5 mL of the Ellman’s reagent and 1 mL of diluted sulphosalicylic acid. The absorbance was read at 412 nm and the GSH concentration in the samples was estimated by extrapolation from the GHS standard curve. GST activity was determined by the method of Habig et al. [34]. Briefly, the assay mixture contained of 30 μL of reduced GSH (0.1 M), 2.79 mL phosphate buffer (0.1 M, pH 6.5), 150 μL of CDNB (3.37 mg/mL) and 30 μL of tissue sample. The absorbance of the reaction mixture was measured at 340 nm against the blank after 60 seconds. The activity of the enzyme in the PMF was estimated using the following equation:
Where: 9.6 is the molar extinction coefficient of CDNB (mmolcm−1) and 0.03 is the volume of tissue sample in mL. Lipid peroxidation was assayed by measuring the thiobarbituric acid reactive (TBAR) products present in the test sample using the procedure of Varshney and Kale [35]. Briefly, the reaction mixture is made up of 1.6 mL Tris-KCl buffer, 0.5 mL of 30% TCA, 0.4 mL of the test sample, 0.5 mL of 0.75% TBA. The temperature of the mixture was raised to 95 °C and maintained at same for 1 h in a water bath. The mixture was then cooled on ice and centrifuged at 3000 rpm. The clear supernatant was collected and the absorbance read against a reference blank of distilled water at 532 nm using a spectrophotometer. Lipid peroxidation in nmole/mg protein was estimated using the following equation:
Where: E532 is the molar extinction coefficient for MDA=1.56 × 105 M−1 cm−1. The concentrations of total cholesterol, HDL- and LDL-cholesterol and triglyceride concentrations were assayed using the CHOD-PAD enzymatic colorimetric method described by Trinder [36] according to the manufacturer’s instruction Randox diagnostic kits, UK [36].
Molecular docking of selected compounds with protein targetsProtein preparationThe crystal structures of androgen receptor (AR) with PDB ID of 1e3g was retrieved from the protein databank (http://www.rcsb.org) and prepared by eliminating existing ligands and water molecules while the absent hydrogen atoms were added using the Autodock v4.2 program, Scripps Research Institute. The search grid was expanded above the target proteins and the parameters of the atomic solution were determined. Polar hydrogen charges of the Gasteiger type were allocated and the non-polar hydrogens were integrated with the carbons and the internal degrees of freedom and torsion were formed, after which the protein was saved in PDBQT format.
Ligand preparationThe structure data file (SDF) formats of testosterone, metolachlor and atrazine were obtained from the PubChem database (www.pubchem.ncbi.nlm.nih.gov) and converted to mol2 chemical format with Open Babel program [37]. The ligands’ alpha carbons were detected when the internal degrees of freedom and torsion were set to zero, and then converted using Autodock tools to PDBQT format.
Molecular dockingDocking of the compound to androgen receptor as well as the assessment of binding affinities was done using Vina GUI (Trott& Olson, 2010). The PDBQT format of the protein and the ligands were dragged into their respective columns. The grid center for docking was detected as X=9.54, Y=21.48, Z=36.52 with the dimensions of the grid box, 65.64×67.134× 96.46 for M3 muscarinic acetylcholine receptor, X=124.98, Y=−17.37, Z=129.67 with the dimensions of the grid box, 58.98× 64.41×99.26 for prostaglandin E2 receptor 3. Subsequently, the program was run and cluster analysis based on root mean square deviation (RMSD) values for starting geometry was conducted and the lowest energy conformation of the more populated cluster was found to be the most accurate solution. The pose with the strongest affinity for each cluster was taken as the representation of this cluster. The compounds were then ranked by their affinity scores. Thereafter, molecular interactions between the protein targets and the compounds that have the highest binding affinity were viewed with Discovery Studio Visualizer, 2020.
Statistical analysisAll data were expressed as mean±SD of six replicates. The data were subjected to one way analysis of variance (ANOVA) and complemented with student’s t-test using sigma plot® statistical software. Values were considered significantly different with respect to control data at p<0.05.
ResultsEffect of ATR-MET administration on plasma creatinine and urea
Table 1 shows the effects of administration of ATR-MET on the levels of plasma creatinine and urea.
ATR-MET-1, ATR-MET-2 and ATR-MET-3 significantly increase the level of plasma creatinine by 92%, 111.7% and 137% respectively compared with control (p<0.05). Plasma urea level significantly increased by the administration of ATR-MET-1, ATR-MET-2 and ATR-MET-3-treated groups by 20%, 31.4% and 42.7% respectively, compared with control (p<0.05).
Effect of ATR-MET administration on the activities of plasma ALP, ALT and AST level bilirubinThe effects of ATR-MET administration on the activities of plasma ALP, ALT and AST and level of bilirubin are presented in Table 2.
Administration of ATR-MET (ATR-MET-1, −2 and −3) significantly increased the plasma ALP activity in the rats by 19%, 31.3% and 38.8% respectively compared with control (p<0.05). Also, Plasma ALT activity was significantly increased by 19.8%, 37.4% and 46.8% by ATR-MET-1, −2 and −3 compared with control (p<0.05). Furthermore, plasma the activity of AST was increased significantly, following ATR-MET-1, −2 and −3 by 24.2%, 30% and 38.6% respectively when compared with control (p<0.05). Similarly, plasma bilirubin also increased significantly by ATR-MET-1, −2 and −3 by 73.3%, 100% and 120% respectively when compared with control (p<0.05). The activity of plasma ACP following administration of ATR-MET is shown in Figure 3. The ACP activity was significantly increased in ATR-MET-1, −2 and 3-treated groups by 71.4%, 87.6% and 108% respectively when compared with the control (p<0.05).
Effect of ATR-MET administration on lipid profileThe effects of ATR-MET treatment on plasma lipids profile in rats is shown in Table 3.
Administration of ATR-MET-1, ATR-MET-2 and ATR-MET-3 significantly increased plasma total cholesterol, LDL-cholesterol and HDL-cholesterol concentrations by 22.4%, 27% and 83.7%; 35%, 35% and 104.5%; and 45.3%, 44% and128% respectively compared to control (p<0.05). Similarly, plasma triglyceride level was significantly increased following administration of Xtravest-1, −2, and −3 by 34.3%, 61.2% and 105.9% respectively compared with control (p<0.05).
The effect of administration of ATR-MET on sperm parametersThe effects of administration of ATR-MET on sperm motility, live/dead ratio, sperm volume, sperm count and daily sperm production (DSP) in rats was shown in Table 4. Administration of ATR-MET at different concentration (ATR-MET-1, −2 and −3) significantly decreased sperm motility, and live/dead respectively by 15%, 42.3% and 54.2%; 3.1%, 15.3% and 40% when compared with control (p<0.05). There were no significant changes in the volume of the sperm in all the groups that were treated with ATR-MET at different concentration when compared with the control. Furthermore, administration of different concentration of ATR-MET-1, −2 and −3 significantly reduced sperm count and daily sperm production (DSP) by 48.7%, 67.4%, and 87.8%; and 10%, 22%, and 46.7% respectively when compared with the control (p<0.05).
The effect of administration of ATR-MET on testicular antioxidantThe effects of administration of ATR-MET on testicular SOD and catalase activities in rats are shown in Table 5. Testicular SOD activity was significantly decreased following administration of ATR-MET-1, −2 and −3 by 48.5%, 100% and 292% respectively compared with control (p<0.05). Also, testicular catalase activity was significantly decreased following administration of ATR-MET-1, −2 and −3 by 59%, 94% and 169% respectively compared with control(p<0.05).
The effect of administration of ATR-MET on testicular GST activity is shown in Figure 4 following treatment with ATR-MET at different concentration (ATR-MET-1, ATR-MET-2 and ATR-MET-3). The activity of GST was significantly decreased by 24.5% in ATR-MET 1, 35.9% in ATR-MET 2, 54.9% in ATR-MET 3-treated group compared with the control (p<0.05). The testicular GSH level following treatment with ATR-MET is shown in Figure 5, while the level of testicular lipid peroxidation (MDA level) following treatment with ATR-MET is shown in Figure 6. The GSH level was significantly decreased by 20%, 42.5% and 119.5% in respectively in ATR-MET1, 2 and 3-treated groups when compared with the control (p<0.05). The MDA level was significantly decreased by 21.6%, 26.1% and 48.5% respectively in ATR-MET 1, 2 and 3-treated groups when compared with the control (p<0.05).
In silico studiesThe result of the in silico study undertaken showed of the binding tendency of atrazine, metolachlor and testosterone for AR to gain an insight into their androgen disruptive effect are shown in Table 6. The result showed a binding affinity of testosterone for AR is −7.7 kcal/mol, while that of atrazine is −5.8 kcal/mol, and that of metolachlor is −10.8 kcal/mol. The amino acid interactions of testosterone and metolachlor on the androgen receptor are shown in Figure 7 and Figure 8 respectively. Figure 7a revealed that testosterone exhibited hydrogen bond interactions with GLN798, LYS847 and SER851 AR, while exhibiting hydrophobic bond interactions with ARG846 and ARG855, while in Figure 8a it was revealed that metolachlor formed hydrogen bonds with SER851 and ARG855, hydrophobic bonds with ARG855, ILE842 and an electrostatic force of attraction with GLU803 of this receptor.
DiscussionHerbicides, including atrazine and metolachlor are produced and used each year around homes and in the agricultural sector. Although a lot of attention has been devoted to elucidating the mechanisms of atrazine toxicity, there are limited reports on the toxicity of its combination with metolachlor. The ATR-MET treated animals showed reduced epididymal and testicular sperm number and sperm motility. These animals also suffered impaired spermatocytogenesis with a concomitant drop in the rate and efficiency of spermatozoa production and the viability of spermatozoa already in storage, in agreement with an earlier study by Simic et al. [39]. The effect of ATR-MET on these sperm parameters indicates that the androgen-secreting capacity in the treated animals was affected. It is known that decrease in sperm count with an associated increase in the percentage of abnormal sperms is associated with infertility in males [40, 41]. Although the adverse effects of atrazine on the reproductive capacity of various animal models are well-reported [10, 42], the effect of ATR-MET, which is a combination of atrazine and metolachlor has not been fully understood. The results obtained from this study indicate that this herbicide could also cause some level of reproductive damage in rats.
The spermatozoa, like most cells have developed a robust antioxidant defense system consisting of enzymes such as catalase, SOD and components like GSH which scavenge and suppress the formation of ROS, while estimation of end products of lipid peroxidation such as MDA is an index of the extent of oxidative damage to cellular structures [43,44]. Oxidative stress happens in tissues when oxidative reactions surpass antioxidant reactions, leading to the loss of the delicate balance between them [45]. The increased level of MDA decreased level of GSH and reduced activities of SOD, catalase and GST in a dose-dependent fashion noticed in the testes homogenates of the ATR-MET-treated animals validate the capacity of the herbicide to cause tissue oxidative damage. This is attributable to the preponderance of polyunsaturated fatty acid in the testis which predisposes it to oxidative stress [46]. Increased MDA level along with altered antioxidant defense machinery such as diminution of GSH level in tissues signals the onset of oxidative stress that may precipitate many peroxidative harm, which is especially a critical issue in the testis because of its vulnerability to oxidative assault [46]. Superoxide dismutase and catalase act as the first line of defense to the cells, while SOD speed up the dismutation of cytotoxic superoxide radicals to H2O2, catalase converts the harmful H2O2 to water and oxygen [44]. The decrease in testicular SOD and catalase activities in animals treated with the herbicide was accompanied with a significant increase in MDA level, as well as reduced GSH level and the activity of GST relative to the control animals. These observations show the ability of the herbicide to overwhelm the antioxidant system of the animals to induce oxidative stress in the testes because over production of ROS and lipid peroxides may cause over utilization of GSH and inhibition of antioxidant enzymes [47,48], while alteration in the activity of testicular SOD might lead to growth arrest and impaired function of the testis and spermatogenesis [49,50]. The decreased activity of catalase in the testis would allow more of the H2O2 to be converted to toxic hydroxyl radicals [51], which might contribute to severe oxidative damage to the cellular membrane of spermatozoa of the atrazine-metolachlor treated animals, resulting in the impaired motility.
The results obtained from this study also showed that ATR-MET induced cardiovascular, hepatic, renal and testicular toxicity in male rats in, with concurrent alterations in testicular antioxidative parameters. The increase in plasma total cholesterol concentration following the administration of ATR-MET corroborates various earlier reports on the effect of toxic agents on plasma level of total cholesterol [52]. This generally suggests deleterious effect on heart conditions including high blood pressure and coronary heart diseases [53]. The elevated plasma HDL-cholesterol, LDL-cholesterol and triglyceride following the administration of ATR-MET also indicate the cardiotoxicity of this herbicide. For example, oxidation of LDL in the vascular endothelium initiates the formation of plaque, a major culprit of cardiovascular diseases. Cholesterol is transported, by LDLs, from where it is produced in the liver to other body tissues, where it is detached from the lipoprotein for subsequent utilization by the cells. LDL-cholesterol is principally responsible for atherosclerotic formation in the blood vessels, especially in the arteries where they cause hardening of the vessels which can also cause heart attack [53]. Therefore, the elevation in plasma LDL-cholesterol concentration occasioned by the administration of ATR-MET suggests that this herbicide may predispose subjects to some cardiovascular diseases. Also, the administration of ATR-MET significantly increased plasma triacylglycerol concentration relative to control, and concurrent increase in plasma triacylglycerol and plasma LDL is said to be a critical coronary risk index [54].
Furthermore, result obtained from this study shows that ATR-MET induced marked increase in renal and hepatic damage biomarkers as evidenced by the significant increase in plasma urea and creatinine levels (renal indices), as well as ALT, AST activities and bilirubin level (hepatic biomarkers). The increased levels of urea and creatinine may indicate acute or chronic kidney disease which may be associated with long duration of symptoms; absence of acute illness, anaemia, hyperphosphataemia, hypocalcaemia [55]. The activities of ALT, AST and the level of bilirubin are useful tools to investigate chemical and drug-induced liver injury, elevation of these indices indicate the liver damaging effect of the administered herbicide in this study [56]. The cytotoxic effect of ATR-MET administration is also revealed by the increased plasma activity of ALP and ACP. These two enzymes are commonly used as a marker enzyme for the integrity of the plasma membrane and endoplasmic reticulum [57,58]. Excess of phosphatases is harmful to the survival of the cells since this may lead to arbitrary hydrolyzation of orthophosphate monoesters [53]. The elevation in the activities of ALP and ACP observed in this study suggests that the integrity of the membrane systems of the animals’ hepatic tissue has been compromised by the administration of the herbicide in a dose-responsive manner, corroborating other hepatic indices that the herbicide could be hepatotoxic.
In addition to in vivo studies, molecular docking was used to characterize the structural binding of atrazine, metolachlor and testosterone with AR to probe the possible endocrine-disrupting ability of these compounds in AR signalling. Like testosterone, metolachlor interacted with the ligand-binding pocket of AR via hydrogen bonding and hydrophobic interactions, but with an additional pi-pi (electrostatic) interaction. The binding energy of metolachlor with AR was higher than that of testosterone, a native ligand of this receptor (Table 8). The amino-acid residue interactions of metolachlor had very high similarity compared to that of testosterone; common hydrogen bonding interaction with amino-acid SER851 and common hydrophobic interaction with ARG855 of AR (Figures 7a and 8a). This structural binding pattern suggested the potential of metolachlor to disrupt AR signalling, which could cause androgen-related reproductive impairment, a similar pattern reported by Beg and Sheikh on the endocrine disruption capacity of di(2-ethylhexyl) phthalate and its metabolites (Beg and Heikh, 2020). This study has used in vivo and in silico evaluations to probe the safety of ATR-MET. The results obtained serve as a basis for further studies on the toxic potentials of ATR-MET in other experimental models, especially in humans.
From the results obtained in this study, it may be concluded that exposure to ATR-MET, a combination of atrazine and metolachlor could promote cardiovascular, renal, hepatic as well as reproductive impairment in the male rats, implying possible similar toxic effects in humans, a concern that deserves further investigation.
Conflict of interestThe authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence this work.
NotesCRediT author statement
ETP: Conceptualization, Supervision, Writing-Review & Editing; AO: Conceptualization, Investigation, Writing-Review & Editing; KEA: Data Curation, Writing-Original draft Preparation, Formal analysis, Writing-Review & Editing; OO: Investigation, Data Curation, Writing-Review & Editing.
References1. Chikoye D, Udensi UE, Lum AF. Evaluation of a new formulation of atrazine and metolachlor mixture for weed control in maize in Nigeria. Crop Prot 2005;24(11):1016-1020
https://doi.org/10.1016/j.cropro.2005.02.011
.
2. Hockley I. Hockley Atrazine+Metolachlor. Hockley Atrazine+Metolachlor; Accessed 30 Jun 2022.
http://hockley.co.uk/crop-protection/herbicides/hockley-atrazine-plus-metolachlor/
.
3. Stoker TE, Laws SC, Guidici DL, Cooper RL. 2000;The effect of atrazine on puberty in male Wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicological Sciences 2000;58(1):50-59
https://doi.org/10.1093/toxsci/58.1.50
.
4. Li J, Li X, Bi H, Li B. The MEK/ERK/CREB signaling pathway is involved in atrazine induced hippocampal neurotoxicity in Sprague Dawley rats. Ecotoxicology and environmental safety 2019;170: 673-681
https://doi.org/10.1016/j.ecoenv.2018.12.038
.
5. Wang J, Du Z, Yu W, Qu S. Detection of seven pesticides in cucumbers using hollow fibre-based liquid-phase microextraction and ultra-high pressure liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography A 2012;1247: 10-17
https://doi.org/10.1016/j.chroma.2012.05.040
.
6. Bouvier G, Blanchard O, Momas I, Seta N. Pesticide exposure of non-occupationally exposed subjects compared to some occupational exposure: a French pilot study. Science of the total environment 2006;366(1):74-91
https://doi.org/10.1016/j.scitotenv.2005.08.016
.
7. Jowa L, Howd R. Should atrazine and related chlorotriazines be considered carcinogenic for human health risk assessment. Journal of Environmental Science and Health, Part C 2011;29(2):91-144
https://doi.org/10.1080/10590501.2011.577681
.
8. Kniewald J, Jakominić M, Tomljenović A, Šimić B, Romac P, Vranešić Đ, et al. Disorders of male rat reproductive tract under the influence of atrazine. Journal of Applied Toxicology:An International Journal 2000;20(1):61-68
https://doi.org/10.1002/(SICI)1099-1263(200001/02)20:1<61::AID-JAT628>3.0.CO;2-3
.
9. Swan SH. Semen quality in fertile US men in relation to geographical area and pesticide exposure. International Journal of Andrology 2006;29(1):62-68
https://doi.org/10.1111/j.1365-2605.2005.00620.x
.
10. Abarikwu SO, Oleribe AL, Mgbudom-Okah CJ, Onuah CL, Chikwendu CS, Onyeike EN. The protective effect of fluted pumpkin seeds against atrazine-induced testicular injury. Drug and Chemical Toxicology 2022;45(2):799-809
https://doi.org/10.1080/01480545.2020.1776723
.
11. Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. Journal of andrology 2001;22(1):142-148
https://doi.org/10.1002/j.1939-4640.2001.tb02164.x
.
12. Gojmerac T, Kartal B, Žurić M, Ćurić S, Mitak M. Serum biochemical and histopathological changes related to the hepatic function in pigs following atrazine treatment. Journal of applied toxicology 1995;15(3):233-236
https://doi.org/10.1002/jat.2550150315
.
13. Maria CS, Moreno J, Lopez-Campos JL. 1987;Hepatotoxicity induced by the herbicide atrazine in the rat. Journal of applied toxicology 1987;7(6):373-378
https://doi.org/10.1002/jat.2550070605
.
14. Vallotton N, Moser D, Eggen RI, Junghans M, Chèvre N. S-metolachlor pulse exposure on the alga Scenedesmus vacuolatus: effects during exposure and the subsequent recovery. Chemosphere 2008;73(3):395-400
https://doi.org/10.1016/j.chemosphere.2008.05.039
.
15. Chen L, Wang D, Tian Z, Di S, Zhang W, Wang F, et al. Comparative toxic responses of male and female lizards (Eremias argus) exposed to (S)-metolachlor-contaminated soil. Environmental Pollution 2017;227: 476-483
https://doi.org/10.1016/j.envpol.2017.05.006
.
16. Buttle JM. Metolachlor transport in surface runoff. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America 1990;19(3):531-538
https://doi.org/10.2134/jeq1990.00472425001900030030x
.
17. Quintaneiro C, Patrício D, Novais SC, Soares AMVM, Monteiro MS. Endocrine and physiological effects of linuron and S-metolachlor in zebrafish developing embryos. Science of the Total Environment 2017;586: 390-400
https://doi.org/10.1016/j.scitotenv.2016.11.153
.
18. Mathias FT, Romano RM, Sleiman HK, de Oliveira CA, Romano MA. Herbicide metolachlor causes changes in reproductive endocrinology of male wistar rats. International Scholarly Research Notices 2012;2012: 1-7
https://doi.org/10.5402/2012/130846
.
19. Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, et al. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology 2006;228(1):98-108
https://doi.org/10.1016/j.tox.2006.08.021
.
20. Committee on Care. Use of Laboratory Animals NI of H (US). D of RR; 2011. Institute of Laboratory Animal Resources (US). National Academies; Guide for the care and use of laboratory animals;
https://nap.nationalacademies.org/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth
.
21. Fang Y, Ni C, Dong Y, Li H, Wu S, Li X, et al. In utero exposure to atrazine disrupts rat fetal testis development. Frontiers in pharmacology 2018;9: 1391
https://doi.org/10.3389/fphar.2018.01391
.
22. Vieira KCDMT, Couto JC, Zanetti E, Junior JMS, Favareto APA. Toxicidade materna e fetal de ratas Wistar expostas ao herbicida metolacloro. Acta Scientiarum-Biological Sciences 2016;38(1):91-98
https://doi.org/10.4025/actascibiolsci.v38i1.29079
.
23. Pant N, Srivastava SP. Correlation of trace mineral concentrations with fructose, γ-glutamyl transpeptidase, and acid phosphatase in seminal plasma of different categories of infertile men. Biological trace element research 2003;93(1):31-38
https://doi.org/10.1385/BTER:93:1-3:31
.
24. Sönmez M, Türk G, Yüce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 2005;63(7):2063-2072
https://doi.org/10.1016/j.theriogenology.2004.10.003
.
25. Wyrobek AJ, Bruce WR. Chemical induction of sperm abnormalities in mice. Proceedings of the National Academy of Sciences 1975;72(11):4425-4429
https://doi.org/10.1073/pnas.72.11.4425
.
26. Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Reproduction 1978;54(1):103-107
https://doi.org/10.1530/jrf.0.0540103
.
27. Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J biol Chem 1949;177(2):751-766.
28. Tietz N, Pruden E, Siggaard-Andersen O. Liver function. In: Burtis A, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. Saunders WB; London, UK: 1994. p. 1354-1374.
29. Wright PJ, Leathwood PD, Plummer DT. Enzymes in rat urine: alkaline phosphatase. Enzymologia 1972;42(4):317-327.
30. Reitman S, Frankel S. Colorimetric methods for aspartate and alanine aminotransferase. Am J Clin Path 1957;28: 55-63
https://doi.org/10.1093/ajcp/28.1.56
.
31. Sinha AK. 1972 Colorimetric assay of catalase. Anal Biochem 1972;47: 389-394
https://doi.org/10.1016/0003-2697(72)90132-7
.
32. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological chemistry 1972;247(10):3170-3175
https://doi.org/10.1016/S0021-9258(19)45228-9
.
33. Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11(3):151-169
https://doi.org/10.1159/000136485
.
34. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of biological Chemistry 1974;249(22):7130-7139
https://doi.org/10.1016/S0021-9258(19)42083-8
.
35. Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. International journal of radiation biology 1990;58(5):733-743
https://doi.org/10.1080/09553009014552121
.
36. Trinder P. A simple Turbidimetric method for the determination of serum cholesterol. Annals of Clinical Biochemistry 1969;6(5):165-166.
37. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. Journal of cheminformatics 2011;3(1):1-14
https://doi.org/10.1186/1758-2946-3-33
.
38. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry 2010;31(2):455-461
https://doi.org/10.1002/jcc.21334
.
39. Simic B, Jakominic M, Romac P, Kniewald J. Effects of atrazine on sperm parameters in rats. Environment 2001;2: 1950-2002.
40. Adewole KE, Attah AF. Antimalarial plants with potential male-factor antifertility properties. Journal of Complementary and Integrative Medicine 2020;17(2):1-29
https://doi.org/10.1515/jcim-2018-0214
.
41. Pandey R, Singh SP. Effects of molybdenum on fertility of male rats. Biometals 2002;15(1):65-72
https://doi.org/10.1023/A:1013193013142
.
42. Aziz RLA, Abdel-Wahab A, El-Ela FIA, Hassan NEHY, El-Nahass ES, Ibrahim MA, et al. Dose-dependent ameliorative effects of quercetin and l-Carnitine against atrazine-induced reproductive toxicity in adult male Albino rats. Biomedicine & pharmacotherapy 2018;102: 855-864
https://doi.org/10.1016/j.biopha.2018.03.136
.
43. Olayinka ET, Adewole KE.
In vivo and in silico evaluation of the ameliorative effect of hesperidin on finasteride-induced testicular oxidative stress in Wistar rats. Toxicology Mechanisms and Methods 2021;31(2):81-89
https://doi.org/10.1080/15376516.2020.1831123
.
44. Adewole KE, Adebayo JO. Antioxidant defense system induced by cysteine-stabilized peptide fraction of aqueous extract of Morinda lucida leaf in selected tissues of Plasmodium berghei-infected mice.
Journal of integrative medicine
2017;15(5):388-397
https://doi.org/10.1016/S2095-4964(17)60354-6
.
45. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevity; 2017.
https://doi.org/10.1155/2017/8416763
.
46. Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Molecular mechanisms in spermatogenesis; 2009. 636
https://doi.org/10.1007/978-0-387-09597-4_9
.
47. Olayinka ET, Adewole KE. Morin attenuates dutasteride/tamsulosin-induced hepatic oxidative stress in rat. Ife Journal of Science 2020;22(1):165-175
https://doi.org/10.4314/ijs.v22i1.16
.
48. Olayinka ET, Ore A. Kolaviron and L-ascorbic acid attenuate chlorambucil-induced testicular oxidative stress in rats. Journal of Toxicology; 2014.
https://doi.org/10.1155/2014/587015
.
49. Feng D, Huang H, Yang Y, Yan T, Jin Y, Cheng X, et al. Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’ testis.
Mutation Research/Genetic Toxicology and Environmental Mutagenesis
2015;792: 35-45
https://doi.org/10.1016/j.mrgentox.2015.09.004
.
50. Ourique GM, Saccol EM, Pes TS, Glanzner WG, Schiefelbein SH, Woehl VM, et al. Protective effect of vitamin E on sperm motility and oxidative stress in valproic acid treated rats. Food and Chemical Toxicology 2016;95: 159-167
https://doi.org/10.1016/j.fct.2016.07.011
.
51. Prahalathan C, Selvakumar E, Varalakshmi P. Remedial effect of DL-α-lipoic acid against adriamycin induced testicular lipid peroxidation. Molecular and cellular biochemistry 2004;267(1):209-214
https://doi.org/10.1023/B:MCBI.0000049385.13773.23
.
52. Szabó A, Szabó-Fodor J, Kachlek M, Mézes M, Balogh K, Glávits R, et al. Dose and exposure time-dependent renal and hepatic effects of intraperitoneally administered fumonisin B1 in rats. Toxins 2018;10(11):465
https://doi.org/10.3390/toxins10110465
.
53. Adebayo JO, Igunnu A, Arise RO, Malomo SO. Effects of co-administration of artesunate and amodiaquine on some cardiovascular disease indices in rats. Food and chemical toxicology 2011;49(1):45-48
https://doi.org/10.1016/j.fct.2010.09.022
.
54. Assmann G, Schulte H. Role of triglycerides in coronary artery disease: lessons from the prospective cardiovascular münster study. The American journal of cardiology 1992;70(19):H10-H13
https://doi.org/10.1016/0002-9149(92)91084-H
.
55. Hilton R. Acute renal failure. BMJ 2006;333: 786-790
https://doi.org/10.1136/bmj.38975.657639.AE
.
56. Abdelaziz RM, Abdelazem AZ, Hashem KS, Attia YA. Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn Schmiedebergs Arch Pharmacol 2020;393: 1405-1417
https://doi.org/10.1007/s00210-020-01843-z
.
57. Adewole KE, Adebayo JO. 2018;Effects of cysteine-stabilized peptide fraction of morinda lucida leaf on selected kidney function indices in mice. Tokai J Exp Clin Med 2018;43(3):90-96.
58. Wright PJ, Plummer DT. 1974;The use of urinary enzyme measurement to detect renal damage caused by nephritic compounds. Biochem Pharmacol 1974;23(1):65-73
https://doi.org/10.1016/0006-2952(74)90314-1
.
59. Beg MA, Sheikh IA. Endocrine disruption: structural interactions of androgen receptor against Di (2-ethylhexyl) phthalate and its metabolites.
Toxics
2020;8(4):115
https://doi.org/10.3390/toxics8040115
.
Figure 3Influence of ATR-MET treatments on plasma acid phosphatase (ACP) activity in rats: TR-MET 1=67.5 mg Atrazine+37.5 mg Metolachlor; ATR-MET 2=135 mg Atrazine+75 mg Metolachlor; ATR-MET 3=270 mg Atrazine+ 150 mg Metolachlor. *Significantly different from the control, p<0.05. 
Figure 4Influence of ATR-MET treatments on testicular glutathione-S-transferase (GST) activity in rats.: ATR-MET 1 =67.5 mg atrazine+37.5 mg metolachlor; ATR-MET 2=135 mg atrazine+75 mg metolachlor; ATR-MET 3=270 mg atrazine +150 mg metolachlor; The values are Means±SD (range) for five rats in each group. *Significantly different from the control, p<0.05. 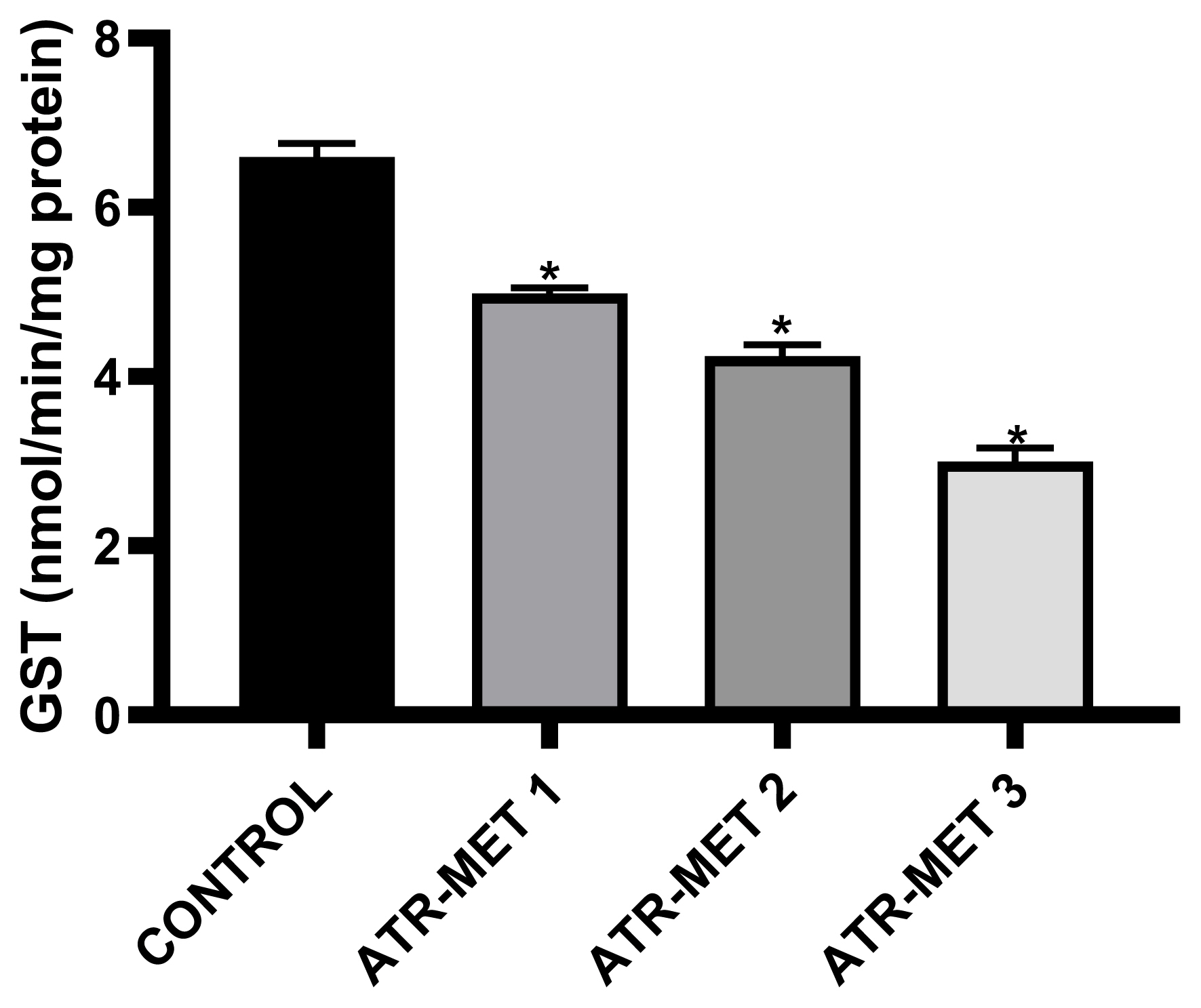
Figure 5Influence of ATR-MET treatments on testicular reduced Glutathione (GSH) in Rats.: ATR-MET 1=67.5 mg atrazine+37.5 mg metolachlor; ATR-MET 2=135 mg atrazine+75 mg metolachlor; ATR-MET 3=270 mg atrazine+150 mg metolachlor. *Significantly different from the control, p<0.05. 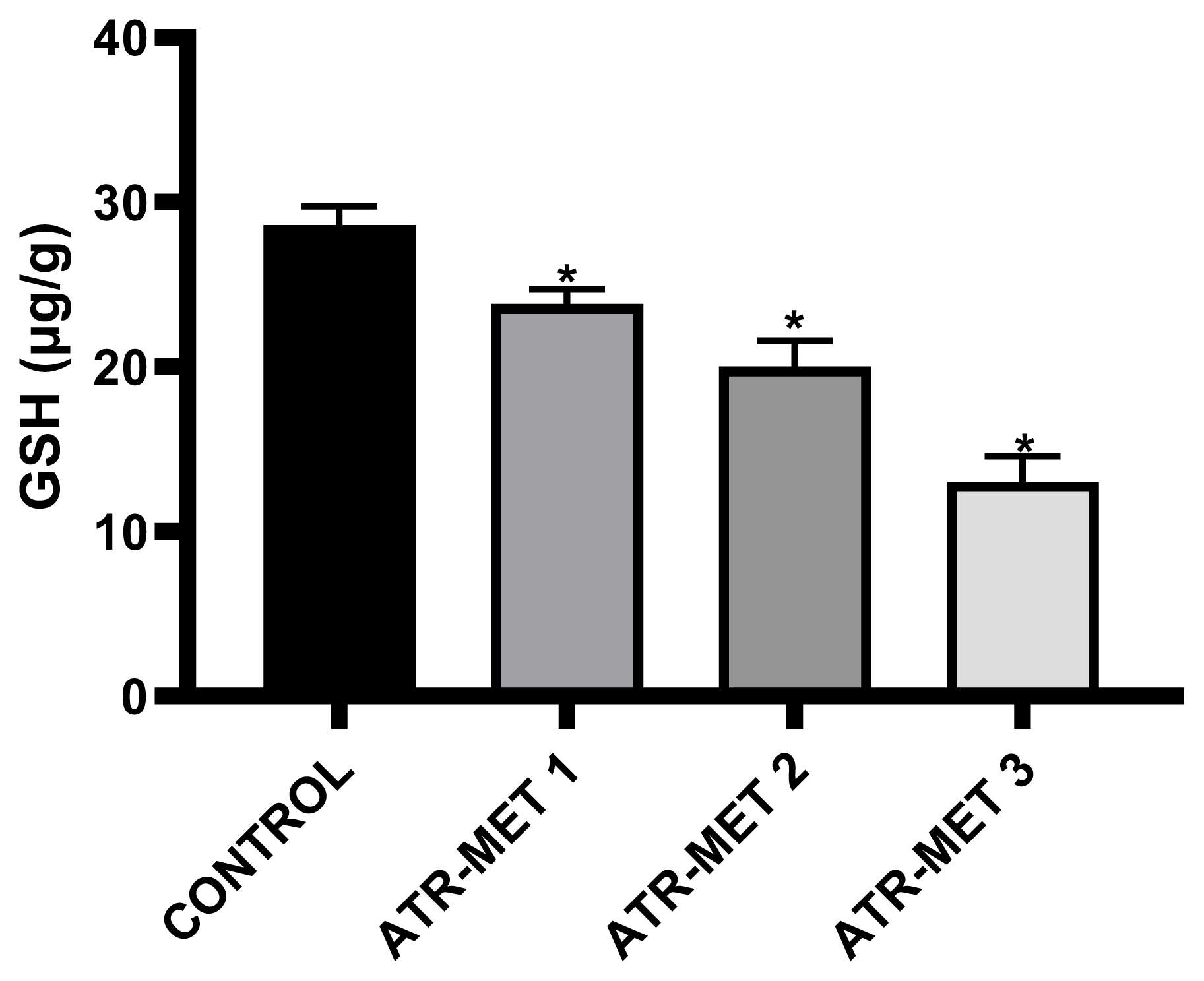
Figure 6Influence of ATR-MET treatments on testicular lipid peroxidation (MDA) in Rats.: ATR-MET 1=67.5 mg atrazine+37.5 mg metolachlor; ATR-MET 2=135 mg atrazine+75 mg metolachlor; ATR-MET3=270 mg atrazine+150 mg metolachlor. *Significantly different from the control, p<0.05. 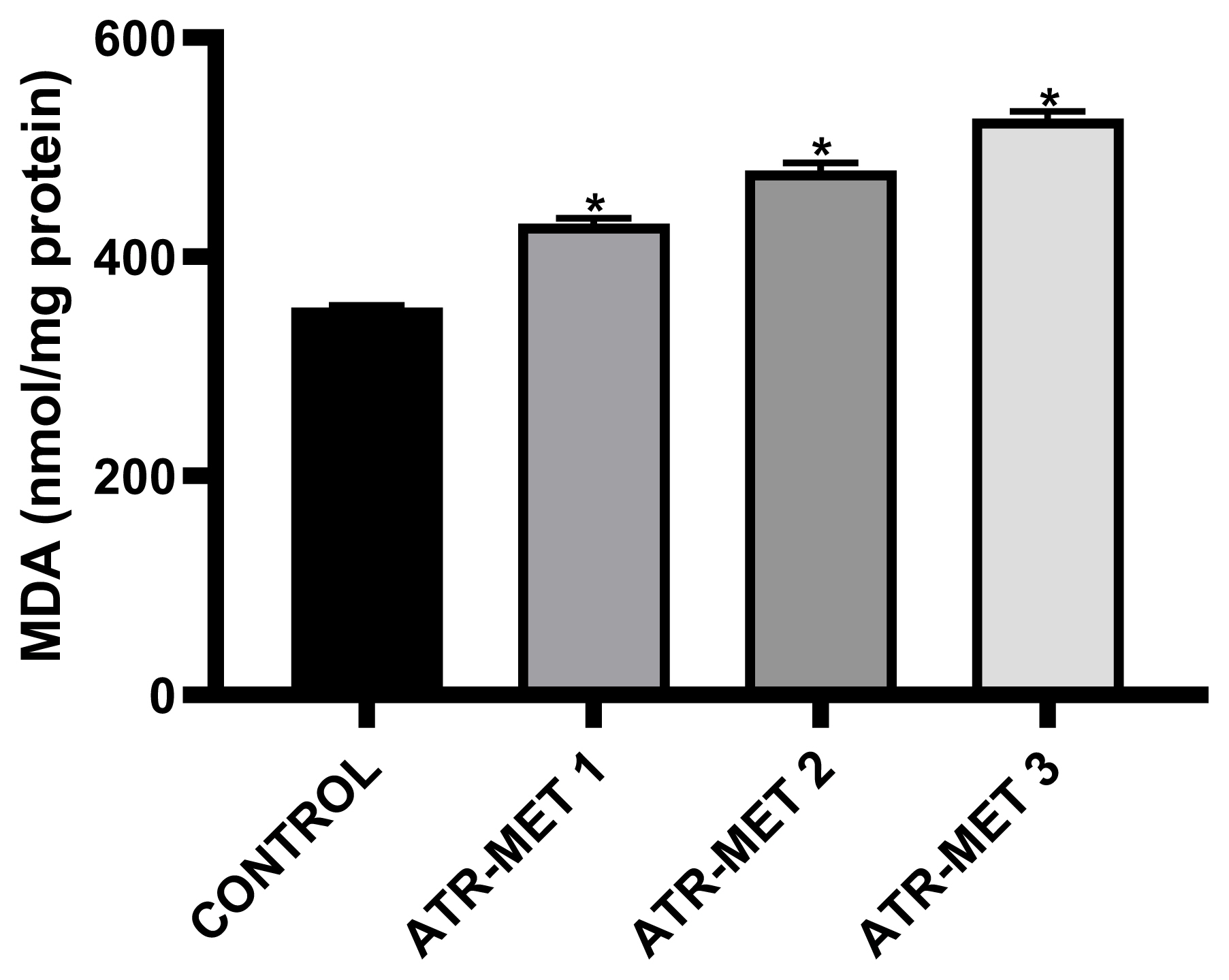
Figure 7Interaction between amino acids in the binding site of androgen receptor and (a) testosterone in 3D and (b) testosterone in 2D 
Figure 8Interaction between amino acids in the binding site of androgen receptor and (a) metolachlor in 3D and (b) metolachlor in 2D. 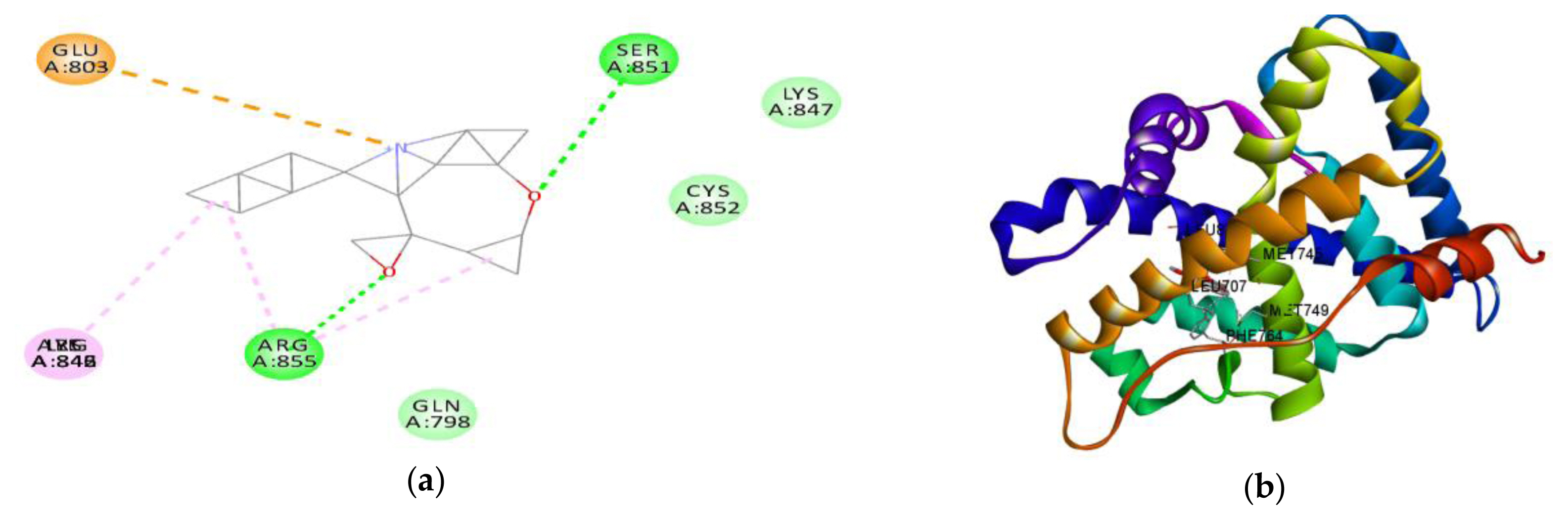
Table 1Effects of ATR-MET treatments on plasma creatinine, urea in rats.
Table 2Effects of administration of Xtravest® on the activities of plasma alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and level bilirubin in rats.
Table 3Effects of administration of ATR-MET on plasma lipids profile in rats.
Table 4Effects of administration of ATR-MET on sperm motility, live/dead ratio, sperm volume, sperm count and daily sperm production (DSP) in rats
Table 5Effects of administration of ATR-MET on superoxide dismutase and catalase activities in rats.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||