AbstractThe use of herbicides for increased food production may pose risk to non-target organisms. This study evaluated the acute toxicity, genotoxic, biochemical, and histological biomarkers of subacute concentrations of paraquat and glyphosate in Oreochromis niloticus (Nile Tilapia) for 28 days following standard methods. Glyphosate (96 hLC50 value-1.23 mg/L) was 9x more toxic than paraquat (96 hLC50 value-11.20 mg/L) against O. niloticus. Average micronucleated cells were significantly higher in the erythrocytes of O. niloticus exposed to the higher (1.12 mg/L) concentration of paraquat at day 14, both subacute concentrations of paraquat at day 28, and lower concentration (0.01 mg/L) of glyphosate at days 14 and 28 compared to the other treatments and controls. Biochemical biomarkers (MDA and GST) activities were significantly higher at both subacute concentrations of the herbicides in the exposed fish compared to the controls at day 28 only. GSH activity was significantly higher in the 0.11 mg/L paraquat concentration while SOD activity was significantly lower at both subacute concentrations of glyphosate in exposed fish compared to controls at day 28. Histological alterations observed were mild to severe shortening of the gill primary lamellar and hepatic portal inflammation of exposed fish compared to the controls. This study demonstrates the risk to non-target organisms due to herbicides’ run-off from agricultural farmlands into aquatic ecosystems at environmentally relevant or subacute concentrations. Sensitization on the responsible use of pesticides is recommended to promote responsible consumption and production and sustain life below water (United Nations Sustainable Development Goals 12 and 14 respectively).
IntroductionAgriculture in modern times rely on pesticides in order to control pests that pose a threat to crop productivity [1]. Pesticides may contaminate the soil, air, and water with potential adverse effects on non-target aquatic organisms, plants, mammals and soil microorganisms [2]. They can bioaccumulate in living organisms, biomagnify along the food chain and inadvertently affect human health [3]. Majority of modern-day pesticides are synthetic organic chemicals with a mode of action that interfere with metabolic processes in target organisms [4]. Paraquat (1,1′-dimethyl-4-4′-bipyridinium) and its dichloride salt (1,1′-dimethyl-4-4′-bipyridinium dichloride) are commonly used herbicides in defoliating and drying during the gathering of commercial crops [5]. Similarly, glyphosate (N-phosphonomethyl) glycine (commercially sold as roundup or force up) is a systemic non-selective herbicide which is used extensively, particularly in developing countries for weed control [6]. It acts through the inhibition of amino acid and protein synthesis leading to the death of the weed within days of application. However, the toxicity of glyphosate is potentiated through its formulation with surfactants to facilitate penetration into the weeds. Thus, non-target organisms such as fish may be inadvertently exposed to these glyphosate formulations when applied on land and washed off into nearby surface waters via run-off or spray drift [7] eliciting acute and chronic biological effects to aquatic life [8].
Though, several studies have evaluated the acute toxicity of paraquat and glyphosate singly in various fish species [9] as well as their subacute effects using different biomarkers [8]. Similarly, several studies have evaluated various biomarkers of toxicity of paraquat and glyphosate singly in Nile Tilapia (O. niloticus). Specifically, for paraquat, studies with O. niloticus have reported 96 hLC50 value of 11.84 mg/L [10] with dose-dependent gill pathologies at exposure levels of 12 mg/L and 14.20 mg/L, LC50 value of 20 mg/L as well as liver and gonadal pathologies at 0.5 mg/L at varying temperatures [11] as well as 96 hLC50 value of 17.49 μL/L [12] of paraquat. For glyphosate, studies using O. niloticus have shown varying adverse effects (such as 96 hLC50 value of 16.8 ppm with gill, liver and kidney pathologies [13], gill, liver and kidney pathologies at sublethal concentrations (5 and 15 ppm) [14], alteration in enzyme activities at 1.2 mg/L [15], gill, liver and kidney pathologies [16], nuclear abnormalities [17] at 17.2 mg/L, antioxidant enzymes (SOD, GSH, CAT) activity reduction, among other biomarkers at different concentrations (0.2, 0.8, 4 and 16 mg/L) for 80 days [18], alterations in blood parameters as well as induction of hepatic oxidative stress and DNA damage in the blood at different concentrations (5, 10, 20, 30 and 40 mg/L) Acar et al. [19] of glyphosate.
However, there are scanty studies which evaluate the comparative genotoxic, biochemical and histological effects of the herbicides, paraquat and glyphosate on the Nile Tilapia (O. niloticus) and at the concentrations used in this study. O. niloticus (Linnaeus, 1758) is a deep-bodied fish with cycloid scales. It is a plankton-feeding omnivorous species native to Africa but it is cultured worldwide [20]. Growth retardation and harmful effects at population and community levels may result from micronuclei formation which is marked by alterations in erythrocyte nuclei [21]. Biochemical biomarkers such as catalase (CAT), superoxide dismutase (SOD), glutathione-s-transferases (GST), reduced glutathione (GSH), malondialdehyde (MDA), an index of lipid peroxidation have been used to assess toxic effects in animals exposed to pollutants [22,23]. Histopathological changes can be used as biomarkers of the effects of anthropogenic pollution on organisms and are compatible as indicators of ecosystem health [24].
Consequently, the study aim was to assess the effects of subacute levels of paraquat and glyphosate on genotoxic, biochemical and histological biomarkers in O. niloticus with a view to evaluate potential risk to non-target animals such as fish from diffuse sources of pesticides into aquatic ecosystems.
Materials and MethodsTest compoundsThe test compounds utilized in this study were herbicides-paraquat and glyphosate which were purchased from a chemical vendor at Ojota, Lagos, Nigeria. Paraquat (paraforce – active ingredient 200 g W.C. in 1 L) and glyphosate (Force Up-360 g glyphosate/L (in the form of 480 g/L Glyphosate-isopropylamine, salt) [25]. A stock solution of 1 g/L was prepared for each herbicide (paraquat: 5 mL in 1 L of dechlorinated tap water=1 g/L; glyphosate: 2.78 mL in 1 L of dechlorinated tap water=1 g/L) from which working solutions were computed.
Collection and acclimatization of test animalThe Nile Tilapia, O. niloticus (Actinopterygii, Perciformes, Cichlidae) juveniles (length: 15.2–18.5 cm) were purchased from an aquaculture farm in Ayobo, Lagos, Nigeria. They were transported in an open 25 L plastic container to the Environmental Toxicology laboratory at the University of Lagos. The fishes were acclimatized to the laboratory environment in a cylindrical plastic tank (60 L) three-quarter filled with dechlorinated tap water for 7 days [25]. The fishes were fed with Coppens feed (2 mm) twice daily. The dechlorinated tap water was changed every 24 h to prevent buildup of waste metabolites and putrefaction of food materials.
Experimental design for acute toxicity studies of paraquat and glyphosate against O. niloticusTests were conducted to obtain the range of concentrations ideal for the definitive tests. Following this, definitive tests were conducted in which five active O. niloticus were randomly introduced into the test tanks containing varying concentrations of paraquat, glyphosate and control (dechlorinated tap water only). Each treatment was duplicated, giving a total of 10 fishes per treatment, including control (without treatment). The concentrations of test compounds were as follows: paraquat-8, 10, 12, 14 and 16 mg/L; glyphosate-0.5, 1.0, 1.5, 2.0 and 2.5 mg/L and control. The bioassay tanks were covered with a net to prevent fish from escaping. Mortality was assessed once in 24 h over the 96 h duration of the experiment [26].
Experimental design for subacute toxicity studiesFor the subacute toxicity studies, eight O. niloticus were exposed in triplicates to subacute concentrations (1/10th and 1/100th of 96 h LC50 values) of paraquat and glyphosate as follows: paraquat: 1.10 mg/L and 0.11 mg/L; glyphosate: 0.10 mg/L and 0.01 mg/L and control. The test media were changed every 72 h following a static renewal bioassay procedure [25]. At 14 and 28 d post-exposure, blood was obtained from O. niloticus selected from the test media and control for genotoxicity studies while gills and liver were excised from the same fishes following euthanization [27] for biochemical and histological studies.
Evaluation of genotoxic biomarkers in O. niloticus exposed to paraquat and glyphosate
O. niloticus juveniles (length range: 15.2–18.5 cm) were exposed to subacute concentrations (1/10th and 1/100th of 96 h LC50 value) of the test compounds in triplicates for 28 days. Peripheral blood samples were drawn from the posterior caudal vein [25] with the aid of a 2 mL syringe on day 14 and 28 during the exposure period. For each concentration, at days 14 and 28, three fishes were used with one slide prepared per fish. Thereafter, the blood was smeared on a clean glass slide, fixed using 100% ethanol for 20 mins and dried at room temperature for 24 h [28]. Afterwards, Giemsa (10%) stain was applied to the smear for 10 mins. The glass slides were evaluated under a digital light microscope (Leica® DM500, Wetzlar, Germany) at 1000x for frequencies of micronuclei and nuclear aberrations. At each assessment, 1000 cells per fish were analysed totaling 3000 erythrocytes for each group. For scoring the micronuclei, the criteria of [29] was adopted.
Assessment of biochemical biomarkers in O. niloticus exposed to paraquat and glyphosateFollowing the harvest of livers of O. niloticus randomly selected from test and control bioassay tanks, the livers were transported to the University of Lagos Biochemistry laboratory in an ice chest for further analysis. The livers were rinsed in ice cold 1.15% KCL solution, blotted and weighed. Thereafter, the livers were homogenized with 0.1 M phosphate buffer (pH 7.2) in a laboratory mortar. Acid-washed laboratory sand was added and blended in the mortar using a pestle [23]. The homogenate therefrom was centrifuged (2500 rpm) for 15 mins, the supernatant was decanted and stored at −20 °C until analysis. The activities of antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione-s-transferases (GST)), reduced glutathione (GSH), and malondialdehyde (MDA) (an index of lipid peroxidation) were determined as described in Sun and Zigma [30], Sinha et al. [31], Habig et al. [32], Sedlak and Lindsay [33] and Buege and Aust [34] respectively.
Assessment of histological biomarkers in O. niloticus exposed to paraquat and glyphosateFollowing euthanization at designated post-exposure days, dissected gills and liver of O. niloticus were washed with buffered normal saline. Afterwards, they were fixed for 48 h in Bouin’s fluid, dehydrated through serial (70% to 100%) changes of ethanol, cleared in xylene and embedded in paraffin wax [25]. Thereafter the gills and liver were sectioned at 5 to 6 μm, stained with eosin and haematoxylin then analysed with a digital light microscope (XSZ-801BN model, China) coupled with 12.1 mega pixels camera (Casio, EX-Z450, Japan) [25].
Statistical analysisThe dose-response data for the 96 h toxicity tests was analyzed using probit analysis [35]. One-way analysis of variance (ANOVA) and Least Significant difference (LSD) test was used to analyze statistical differences between the mean of the genotoxicity (micronuclei, binucleated and blebbed cells frequency) and biochemical biomarkers activity in O. niloticus exposed to subacute concentrations of paraquat and glyphosate at days 14 and 28. The results were deemed significant at p<0.05. All analyses were conducted using SPSS 20.0 for windows.
ResultsRelative acute toxicity of paraquat and glyphosate against O. niloticusThe median lethal concentration (96 h LC50 value) of paraquat and glyphosate against O. niloticus were 11.20 mg/L and 1.22 mg/L respectively. Glyphosate was estimated to be 9x more toxic than paraquat against O. niloticus.
Genotoxic biomarkers in the erythrocytes of O. niloticus exposed to subacute concentrations of paraquat and glyphosateBinucleated (BN) and blebbed cells were non-significantly higher (p>0.05) at both subacute concentrations of paraquat-exposed O. niloticus compared to control on days 14 and 28. Though, BN cells were non-significantly lower (p>0.05) at day 28 only in the 0.11 mg/L concentration compared to the 1.12 mg/L concentration and control Figure 1A, SI 1. However, micronucleated (MN) cells were significantly higher (p<0.05) at the 1.12 mg/L (higher) concentration only at day 14 and at both subacute concentrations of paraquat-exposed O. niloticus on day 28 compared to control Figure 1A, SI 1.
In glyphosate-exposed O. niloticus, there were no significant differences (p>0.05) between BN and blebbed cells at both subacute concentrations and control on days 14 and 28 Figure 1B, SI 1. However, MN cells were significantly higher (p<0.05) in the erythrocytes of O. niloticus exposed to the lower (0.01 mg/L) concentration when compared to the higher (0.12 mg/L) concentration and control at days 14 and 28 (Figure 1B, SI 1).
Biochemical biomarkers in the liver of O. niloticus exposed to subacute concentrations of paraquat and glyphosateAt day 14, there were no significant differences (p>0.05) between paraquat-exposed and control O. niloticus as well as glyphosate-exposed and control O. niloticus for all hepatic antioxidant enzymes and MDA activities (Figure 2, SI 2). Also, at day 28, there were no significant differences (p>0.05) in CAT and SOD enzymes activity between paraquat-exposed and control O. niloticus (Figures 2C and 2D, SI 2). Similarly, at day 28, GSH and CAT enzymes activity were not significantly different (p>0.05) between glyphosate-exposed and control O. niloticus (Figures 2F and 2I, SI 2).
However, significant differences (p<0.05) were observed in GSH, GST and MDA activities at day 28 between paraquat-exposed and control O. niloticus (Figures 2A, 2B and 2E, SI 2). Specifically, GSH activity was significantly higher (p<0.05) in 0.11 mg/L concentration compared to 1.12 mg/L concentration of paraquat-exposed and control O. niloticus (Figure 2A, SI 2). Further, GST and MDA activities were significantly higher (p<0.05) in both subacute concentrations of paraquat-exposed O. niloticus when compared to controls (Figure 2B and 2E, SI 2).
Similarly, significant differences (p<0.05) were observed in GST, SOD and MDA activities at day 28 between glyphosate-exposed and control O. niloticus (Figures 2G, 2H and 2J, SI 2). Specifically, GST and MDA activities were significantly higher (p<0.05) (Figures 2G and 2J, SI 2) while SOD activity was significantly lower (p<0.05) at both subacute concentrations of glyphosate-exposed O. niloticus compared to control (Figure 2H, SI 2).
Histological biomarkers in O. niloticus exposed to subacute concentrations of paraquat and glyphosatePhotomicrographs of the representative histological sections from fish in each experimental group is shown in Figures 3 and 4. The histological evaluations revealed mild shortening (0.11 mg/L paraquat, 0.12 mg/L glyphosate) (Figures 3b, 3e, 3g and 3j) to severe shortening (1.12 mg/L paraquat, 0.01 mg/L glyphosate) (Figures 3c, 3h and 3i) of the primary lamella in the gills of exposed O. niloticus respectively at days 14 and 28. Though no abnormalities were observed in the gills of 0.01 mg/L glyphosate exposed O. niloticus at day 14 (Figure 3d) and control O. niloticus at days 14 and 28 (Figures 3a and 3f). Portal inflammatory cells were observed at day 14 in 0.12 mg/L glyphosate-exposed O. niloticus only (Figure 4e) and in all paraquat- and glyphosate-exposed O. niloticus at day 28 (Figures 4g–4j). There were no histological abnormalities in the liver of paraquat-exposed and 0.01 mg/L glyphosate-exposed O. niloticus at day 14 (Figures 4b–4d) and control O. niloticus at days 14 and 28 Figures 4a and 4f.
DiscussionThis study evaluated the relative acute toxicity as well as the genotoxic, biochemical and histological biomarkers of exposure to subacute concentrations of the herbicides (paraquat and glyphosate) in O. niloticus (Nile Tilapia). The 96 hLC50 value (11.20 mg/L) of paraquat to O. niloticus in this study is lower than the 96 hLC50 values of 11.84 mg/L and 20 mg/L reported in O. niloticus exposed to paraquat by Babatunde et al. [10] and Figueiredo-Fernandes et al. [11] respectively. Similarly, the 96 hLC50 value (1.22 mg/L) of glyphosate in the current study is lower than the observed LC50 values of 11.30 mg/L [36] and 16.8 ppm [13] in O. niloticus exposed to glyphosate. The observed higher toxicity of glyphosate up to 9x that of paraquat differs with the observations of Ayanda et al. [9] who stated paraquat toxicity up to ~8x that of glyphosate against Clarias gariepinus. Similarly, in an immobility assay with Daphnia magna, paraquat (OSAQUAT) was observed to be ~14x more toxic when compared to glyphosate (RON-DO) based on the 48 hEC50 values [37]. Glyphosate and paraquat have been observed to be moderately toxic and very toxic respectively to aquatic organisms [37,38]. The higher toxicity of glyphosate compared to paraquat in this study may be due to the “extreme” stability of the former in sterile water in the laboratory [37,39]. The differential toxicities of the two herbicides against O. niloticus can be attributed to their different methods of action, fish species, herbicide formulation and life stage [25,40].
A chemical genotoxic agent’s action may result to an increase in micronucleus and nuclear abnormalities occurrence [41]. A vast amount of chemicals can impede the DNA synthesis of an exposed organism and this can bring about nuclear aberrations [42]. The development of these aberrations represents a way to remove from the cell nucleus any elaborated genetic material [43]. The dose-dependent genotoxic biomarkers (nuclear aberrations) observed in the paraquat-exposed fishes especially for MN differs from the observations of Amaeze et al. [44] who observed minimal nuclear abnormalities including no MN at paraquat exposures of 0.1 mg/L (102.84 μg/L). However, the study results corroborate the observations of Oladokun et al. [25] who reported a significant (p<0.05) dose-dependent increase in micronuclei in the red blood cells of the African sharptooth catfish (C. gariepinus) exposed to sublethal concentrations of paraquat at day 28. On the other hand, the observed significant increase in MN in the glyphosate-exposed O. niloticus, though not dose-dependent corroborates the findings of Samantha et al. [17] and Acar et al. [19] who reported nuclear abnormalities in O. niloticus exposed to 17.2 mg/L and different concentrations 5, 10, 20, 30 and 40 mg/L respectively of glyphosate. Several studies have reported pollutants which induce nuclear aberrations in fish tissues [45,46]. Whilst the mechanisms initiating these nuclear aberrations are not fully described, these aberrations are viewed as signs of genotoxic damage, hence, they can match the scoring of these parameters (micronuclei, blebbed nuclei and binucleated cells) during normal genotoxicity surveys [21,47].
Aquatic ecosystems are usually affected by numerous pesticides from various sources [48]. Perturbations at the biochemical and cellular levels are amongst the subtle biological responses observed from exposure of fishes to pollutants [49]. Herbicides can potentially elicit reactive oxygen species (ROS) in living organisms causing oxidative stress in non-target organisms [50]. Defensive mechanisms have emerged from fish and other vertebrates to counter the deleterious ROS effects resulting from various xenobiotics metabolism [51]. CAT and SOD enzymes possess similar roles [52]. SOD is a metalloenzyme that plays an important antioxidant role. It is the major protection countering the toxic superoxide radicals effect in organisms enabling the conversion of superoxide radicals to H2O2 and H2O [53]. Catalase facilitates the removal of H2O2, which metabolizes to molecular oxygen and water [54]. The observed induction of lipid peroxidation (indicated by increased MDA levels) and most antioxidant enzymes in this study especially at day 28 agrees with previous studies reporting alteration in enzyme activities in glyphosate-exposed O. niloticus at concentrations of 1.2 mg/L [15], induction of hepatic oxidative stress at 5, 10, 20, 30 and 40 mg/L [19] and reduction in antioxidant enzymes activity at 0.2, 0.8, 4 and 16 mg/L [18] of glyphosate. Further, this study revealed a significant decrease in SOD level at day 28 following exposure O. niloticus to subacute concentrations of glyphosate. This could be a reaction to the elevated ROS induced by the toxic effects of herbicides [55]. The observed significantly increased GST levels in the herbicides concentrations and significantly increased GSH level in the lower paraquat concentration at day 28 only contrasts with the findings of Moustafa et al. [56] who observed a significant reduction in GSH and GST levels in C. gariepinus exposed to glyphosate. The major function of GSH is to protect cells against oxidative stress due to free radicals through reductive detoxification of reactive intermediates like H2O2 [57]. On the other hand, GSTs potentiates the coupling of reduced GSH with electrophilic metabolites. Also, they are involved in depuration of reactive intermediates and oxygen radicals [54]. Studies have shown that activities of these enzymes may be potentiated in the liver of fish exposed to various pollutants [50].
Histology studies are regarded as a sensitive endpoint in detecting organ toxicity to xenobiotics [58] with capabilities of revealing detailed information regarding the acute and chronic effects of toxicants on targeted organs which may not be detected by functional biomarkers [59]. Fish gills are the main place for ion exchange with the environment, and also, the main channel of pesticide penetration, this is because they are in constant contact with water [60]. The histological alterations observed in this study ranging from mild to severe lamellae necrosis especially at day 28 in the herbicides-exposed fishes agrees with the observations of liver and gonadal pathologies as well as gill pathologies in O. niloticus exposed to paraquat at 0.5 mg/L [11] as well as 12 mg/L and 14.20 mg/L [10] respectively. Similarly, the study findings agree with previous studies observations in glyphosate-exposed O. niloticus of gill, liver and kidney pathologies at 5, 12 and 16.8 ppm [13,14] as well as at 17.2 mg/L [16]. Several studies have revealed alterations in O. niloticus liver elicited by different toxic chemicals [61]. Fusion of secondary lamellae as a result of exposure to pesticides appears to have a protective role in diminishing the affected gill surface; this response slows down the penetration of toxic and may result in fish choking [62, 63].
ConclusionsThis study revealed significant biomarkers of subacute concentrations of paraquat and glyphosate in O. niloticus. The novelty of this study is the comparative assessment of multiple biomarkers at varying levels of biological organization (from cell to whole system level) of two (2) commonly used herbicides (paraquat and glyphosate) at subacute concentrations (near environmentally relevant concentrations). The observed toxicities may pose ecological risk through potential bioaccumulation of the herbicides in non-target aquatic organisms and biomagnification through the food chain. Hence, studies and advocacies on risk of herbicides use with potential attendant impact on non-target organisms are essential [64]. Appropriate and irregular herbicides use are advised so that the advantageous effects of these chemicals will be achieved. These will promote responsible consumption and production and sustain life below water (United Nations Sustainable Development Goals 12 and 14 respectively).
AcknowledgementThe authors thank Mr. Monday Akapo and Mr. Kasali of the Department of Marine Sciences, Faculty of Science, University of Lagos for their technical assistance in handling the Nile tilapia.
NotesEthics statement
This study followed the principles in the Declaration of Helsinki on the humane treatment of animals used in research (http://www.wma.net/en/30publications/10policies/a18/) and the principles in the American Veterinary Medical Association Guidelines for the euthanasia of animals [27].
NotesAvailability of data and material
All data associated with this manuscript are included here and in the supplementary information. Original raw data utilized for the statistical analysis of study data are available on request.
NotesCRediT author statement
OAA: Investigation, Data curation, Writing-original draft preparation, Writing-review and editing, Funding acquisition; TOS: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing-review and editing, Formal analysis, Resources; KAK; Methodology, Writing-review and Editing, Resources
References1. Mahmood I, Imadi SR, Shazadi K, Gul A, Hakeem KR. Effects of pesticides on environment. In: Hakeem K, Akhtar M, Abdullah S, editors. Plant, Soil and Microbes. Springer; Cham: 2016. p. 253-269
https://doi.org/10.1007/978-3-319-27455-3_13
.
2. Liang HC, Razaviarani V, Buchanan I. Pesticides and herbicides. Water Environment Research 2013;85(10):1601-1644
https://doi.org/10.2175/106143013X13698672322660
.
3. Thakur M, Pathania D. Chapter 12: Environmental fate of organic pollutants and effect on human health. Abatement of Environmental Pollutants. Elsevier; 2020. p. 245-262
https://doi.org/10.1016/B978-0-12-818095-2.00012-6
.
4. Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. Pesticide toxicity: a mechanistic approach. EXCLI journal 2018;17: 1101-1136
https://doi.org/10.17179/excli2018-1710
.
5. Mbuk RO, Ato RS, Nkpa NN. The role of paraquat (1, 1,-dimethyl-4, 4-bipyridinium chloride) and glyphosate (N-phosphonomethyl glycine) in translocation of metal ions to subsurface soils. Pak J Anal Environ Chem 2009;10(1&2):19-24.
6. Romero DM, de Molina MCR, Juárez ÁB. Oxidative stress induced by a commercial glyphosate formulation in a tolerant strain of Chlorella kessleri
. Ecotoxicology and Environmental Safety 2011;74(4):741-747
https://doi.org/10.1016/j.ecoenv.2010.10.034
.
7. Stanley J, Preetha G. Pesticide toxicity to fishes: exposure, toxicity and risk assessment methodologies. Pesticide Toxicity to Non-target Organisms; Springer; Dordrecht: 2016. 411-497
https://doi.org/10.1007/978-94-017-7752-0_7
.
8. Ayanda OI, Oniye SJ, Auta JA, Ajibola VO, Bello OA. Responses of the African catfish Clarias gariepinus to long-term exposure to glyphosate-and paraquat-based herbicides. African Journal of Aquatic Science 2015;40(3):261-267
https://doi.org/10.2989/16085914.2015.1074882
.
9. Ayanda OI, Oniye SJ, Auta J, Ajibola VO. Acute toxicity of glyphosate and paraquat to the African catfish (Clarias gariepinus, Teugels 1986) using some biochemical indicators. Tropical Zoology 2015;28(4):152-162
https://doi.org/10.1080/03946975.2015.1076661
.
10. Babatunde MM, Oladimeji AA, Balogun JK. Acute toxicity of gramoxone to Oreochromis niloticus (Trewavas) in Nigeria. Water, Air, and Soil Pollution 2001;131(1):1-10
https://doi.org/10.1023/A:1011959514500
.
11. Figueiredo-Fernandes A, Fontaínhas-Fernandes A, Rocha E, Reis-Henriques MA. The effect of paraquat on hepatic EROD activity, liver, and gonadal histology in males and females of Nile Tilapia, Oreochromis niloticus, exposed at different temperatures. Archives of Environmental Contamination and Toxicology 2006;51(4):626-632
https://doi.org/10.1007/s00244-005-0208-3
.
12. Gil Diaz D, Navarrete Rodríguez G, Castañeda Chavez MDR, Galaviz Villa I, Sosa Villalobos CA. Paraquat’s herbicide acute toxicity in Oreochromis niloticus (Cichlidae) and Macrobrachium olfersii (Palaemonidae). Acta Biológica Colombiana 2021;26(2):178-185
https://doi.org/10.15446/abc.v26n2.84792
.
13. Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P. Histopathological effects of roundup, a glyphosate herbicide, on Nile tilapia (Oreochromis niloticus). Science Asia 2002;28(3):121-7
https://doi.org/10.2306/scienceasia1513-1874.2002.28.121
.
14. Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P. Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus). Environmental Toxicology: An International Journal 2003;18(4):260-267.
15. Dey S, Samanta P, Pal S, Mukherjee AK, Kole D, Ghosh AR. Integrative assessment of biomarker responses in teleostean fishes exposed to glyphosate-based herbicide (Excel Mera 71). Emerging Contaminants 2016;2(4):191-203
https://doi.org/10.1016/j.emcon.2016.12.002
.
16. Samanta P, Kumari P, Pal S, Mukherjee AK, Ghosh AR. Histopathological and ultrastructural alterations in some organs of Oreochromis niloticus exposed to glyphosate-based herbicide, Excel Mera 71. Journal of microscopy and ultrastructure 2018;6(1):35-43
https://doi.org/10.4103/JMAU.JMAU_8_18
.
17. Samanta P, Pal S, Mukherjee AK, Senapati T, Jung J, Ghosh AR. Assessment of adverse impacts of glyphosate-based herbicide, Excel Mera 71 by integrating multi-level biomarker responses in fishes. International Journal of Environmental Science and Technology 2019;16(10):6291-6300
https://doi.org/10.1007/s13762-018-2013-3
.
18. Zheng T, Jia R, Cao L, Du J, Gu Z, He Q, et al. Effects of chronic glyphosate exposure on antioxdative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2021;239: 108878
https://doi.org/10.1016/j.cbpc.2020.108878
.
19. Acar Ü, İnanan BE, Navruz FZ, Yılmaz S. Alterations in blood parameters, DNA damage, oxidative stress and antioxidant enzymes and immune-related genes expression in Nile tilapia (Oreochromis niloticus) exposed to glyphosate-based herbicide. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2021;249: 109147
https://doi.org/10.1016/j.cbpc.2021.109147
.
20. Zengeya TA, Booth AJ, Bastos AD, Chimimba CT. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environmental Biology of Fishes 2011;92(4):479-489
https://doi.org/10.1007/s10641-011-9865-4
.
21. Caliani I, Rodríguez LP, Casini S, Granata A, Zagami G, Pansera M, et al. Biochemical and genotoxic biomarkers in Atherina boyeri to evaluate the status of aquatic ecosystems. Regional Studies in Marine Science 2019;28: 100566
https://doi.org/10.1016/j.rsma.2019.100566
.
22. El-Sayed YS, Saad TT, El-Bahr SM. Acute intoxication of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effects. Environmental Toxicology and Pharmacology 2007;24(3):212-217
https://doi.org/10.1016/j.etap.2007.05.006
.
23. Amaeze NH, Adeyemi RO, Adebesin AO. Oxidative stress, heats shock protein and histopathological effects in the gills of African catfish, Clarias gariepinus induced by bridge runoffs. Environmental Monitoring and Assessment 2015;187(4):1-16
https://doi.org/10.1007/s10661-015-4390-0
.
24. Velkova-Jordanoska L, Kostoski G. Histopathological analysis of liver in fish (Barbus meridionalis petenyi Heckel) in reservoir Trebeništa. Natura Croatica: Periodicum Musei Historiae Naturalis Croatici 2005;14(2):147-153.
25. Oladokun EI, Sogbanmu TO, Anikwe JC. Sublethal concentrations of dichlorvos and paraquat induce genotoxic and histological effects in the Clarias gariepinus
. Environmental Analysis, Health and Toxicology; 2020. 35(3):
https://doi.org/10.5620/eaht.2020013
.
26. OECD Test No 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing; Paris: 2019.
https://doi.org/10.1787/9789264069961-en
.
27. American Veterinary Medical Association (AVMA). AVMA guidelines for the euthanasia of animals 2013;102.
28. Rezende KFO, Santos RM, Borges JCS, Salvo LM, da Silva JRMC. Histopathological and genotoxic effects of pollution on Nile Tilapia (Oreochromis niloticus, Linnaeus, 1758) in the Billings Reservoir (Brazil). Toxicology Mechanisms and Methods 2014;24(6):404-411
https://doi.org/10.3109/15376516.2014.925020
.
29. Al-Sabti K, Metcalfe CD. Fish micronuclei for assessing genotoxicity in water. Mutation Research/Genetic Toxicology 1995;343(2–3):121-135
https://doi.org/10.1016/0165-1218(95)90078-0
.
30. Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Analytical biochemistry 1978;90(1):81-89
https://doi.org/10.1016/0003-2697(78)90010-6
.
31. Sinha AK. Colorimetric assay of catalase. Analytical biochemistry 1972;47(2):389-394
https://doi.org/10.1016/0003-2697(72)90132-7
.
32. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of biological Chemistry 1974;249(22):7130-7139
https://doi.org/10.1016/S0021-9258(19)42083-8
.
33. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical biochemistry 1968;25: 192-205
https://doi.org/10.1016/0003-2697(68)90092-4
.
34. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology. Academic press; 1978. 52: p. 302-310
https://doi.org/10.1016/S0076-6879(78)52032-6
.
35. Finney DJ. Probit analysis. 3rd ed. Cambridge University Press; Cambridge UK: 1971. p. 333.
36. Fai PBA, Mbida M, Demefack JM, Yamssi C. Potential of the microbial assay for risk assessment (MARA) for assessing ecotoxicological effects of herbicides to non-target organisms. Ecotoxicology 2015;24(9):1915-1922
https://doi.org/10.1007/s10646-015-1527-4
.
37. Alberdi JL, Saenz ME, Di Marzio WD, Tortorelli MC. Comparative acute toxicity of two herbicides, paraquat and glyphosate, to Daphnia magna and D. spinulata
. Bull Environ Contam Toxicol 1996;57(2):229-235
https://doi.org/10.1007/s001289900180
.
38. Tortorelli MC, Hernandez DA, Vázquez GR, Salibián A. Effects of paraquat on mortality and cardiorespiratory function of catfish fry Plecostomus commersoni. Archives of environmental contamination and toxicology 1990;19(4):523-529
https://doi.org/10.1007/BF01059071
.
39. Bronstad JO, Friestad HO. Behaviour of glyphosate in the aquatic environment. Herbicide glyphosate. In: Grossbard E, Atkinson D, editors. 1985. p. 200-205.
40. World Health Organization (WHO). Glyphosate Environmental Health Criteria No 159; Geneva, Switzerland: 1994.
https://wedocs.unep.org/20.500.11822/29463
.
41. Salvagni J, Ternus RT, Fuentefria AM. Assessment of the genotoxic impact of pesticides on farming communities in the countryside of Santa Catarina State, Brazil. Genet Mol Biol 2011;34(1):122-126
https://doi.org/10.1590/S1415-47572010005000104
.
42. Ventura BC, Angelis DF, Marin-Morales MA. Mutagenic and genotoxic effects of the atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic Biochem Phys 2008;90(1):42-51
https://doi.org/10.1016/j.pestbp.2007.07.009
.
43. Shimizu N, Itoh N, Utiyama H, Wahl GM. Selective entrapment of extrachromosomally amplified DNA by nuclear budding and micronucleation during S phase. The Journal of cell biology 1998;140(6):1307-1320
https://doi.org/10.1083/jcb.140.6.1307
.
44. Amaeze NH, Komolafe BO, Salako AF, Akagha KK, Briggs TMD, Olatinwo OO, Femi MA. Comparative assessment of the acute toxicity, haematological and genotoxic effects of ten commonly used pesticides on the African Catfish. Clarias gariepinus Burchell 1822 Heliyon 2020;6(8):e04768
https://doi.org/10.1016/j.heliyon.2020.e04768
.
45. Mumuni AA, Sogbanmu TO. Embryotoxic, developmental and genotoxic evaluations of a endosulfan and deltamethrin mixture on the African sharptooth catfish (Clarias gariepinus). West African Journal of Applied Ecology 2018;26(1):1-10.
46. Talapatra SN, Banerjee SK. Detection of micronucleus and abnormal nucleus in erythrocytes from the gill and kidney of Labeo bata cultivated in sewage-fed fish farms. Food and chemical toxicology 2007;45(2):210-215
https://doi.org/10.1016/j.fct.2006.07.022
.
47. Strunjak-Perovic I, Coz-Rakovac R, Jadan M. Seasonality of nuclear abnormalities in gilthead sea bream Sparus aurata (L.) erythrocytes. Fish physiology and biochemistry 2009;35(2):287-291
https://doi.org/10.1007/s10695-008-9208-3
.
48. Aktar MW, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary toxicology 2009;2(1):1-12
https://doi.org/10.2478/v10102-009-0001-7
.
49. Sandrini JZ, Rola RC, Lopes FM, Buffon HF, Freitas MM, Martins CMG, Rosa CE. Effects of glyphosate on cholinesterase activity of the mussel Pernaperna and the fish Danio rerio and Jenynsia multidentata: In vitro studies. Aquat Toxicol 2013;130: 171-173
https://doi.org/10.1016/j.aquatox.2013.01.006
.
50. Machala M, Petřivalský M, Nezveda K, Ulrich R, Dušek L, Piačka V, Svobodová Z. Responses of carp hepatopancreatic 7-ethoxyresorufin-O-deethylase and glutathione-dependent enzymes to organic pollutants – a field study. Environ Toxicol Chem 1997;16(7):1410-1416
https://doi.org/10.1002/etc.5620160713
.
51. Ortiz-Ordoñez E, Uría-Galicia E, Ruiz-Picos RA, Sánchez Duran AG, Hernández Trejo Y, Sedeño-Díaz JE, López-López E. Effect of Yerbimat herbicide on lipid peroxidation, catalase activity, and histological damage in gills and liver of the freshwater fish Goodea atripinnis. Archives of environmental contamination and toxicology 2011;61(3):443-452
https://doi.org/10.1007/s00244-011-9648-0
.
52. Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2006;143(2):141-149
https://doi.org/10.1016/j.cbpc.2006.01.004
.
53. Kohen R, Nyska A. Invited review: Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic pathology 2002;30(6):620-650
https://doi.org/10.1080/01926230290166724
.
54. Van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental toxicology and pharmacology 2003;13(2):57-149
https://doi.org/10.1016/S1382-6689(02)00126-6
.
55. John S, Kale M, Rathore N, Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. The Journal of nutritional biochemistry 2001;12(9):500-504
https://doi.org/10.1016/S0955-2863(01)00160-7
.
56. Moustafa GG, Shaaban FE, Hadeed AA, Elhady WM. Immunotoxicological, biochemical, and histopathological studies on Roundup and Stomp herbicides in Nile catfish (Clarias gariepinus). Veterinary world 2016;9(6):638-647
https://doi.org/10.14202/vetworld.2016.638-647
.
57. Bukowska B. Effects of 2,4-d and its metabolite 2,4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Comp Biochem Physiol C 2003;135(4):435-441
https://doi.org/10.1016/S1532-0456(03)00151-0
.
58. Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, et al. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol 2002;30(4):507-520
https://doi.org/10.1080/01926230290105695
.
59. Amacher DE, Schomaker SJ, Boldt SE, Mirsky M. The relationship among microsomal enzyme induction, liver weight and histological change in cynomolgus monkey toxicology studies. Food Chem Toxicol 2006;44(4):528-537
https://doi.org/10.1016/j.fct.2005.08.027
.
60. Soare LC, Păunescu A, Maria PC. The morphophysiological, histological, and biochemical response of some nontarget organisms to the Stress Induced by the Pesticides in the Environment; IntechOpen; 2019.
http://dx.doi.org/10.5772/intechopen.84332
.
61. Figueiredo-Fernandes A, Fontaínhas-Fernandes A, Monteiro R, Reis-Henriques MA, Rocha E. Effects of the fungicide mancozeb on liver structure of Nile tilapia, Oreochromis niloticus: assessment and quantification of induced cytological changes using qualitative histopathology and the stereological point-sampled intercept method. Bulletin of Environmental Contamination & Toxicology 2006;76(2.
62. Skidmore JF. Toxicity of zinc compounds to aquatic animals with special reference to fish. The Quarterly Review of Biology 1964;39(3):227-248
https://doi.org/10.1086/404229
.
63. Burton DT, Jones AH, Cairns J. Acute zinc toxicity to rainbow trout (Salmo gairneri): confirmation of the hypothsis that death is related to tissue hypoxia. Journal of the Fisheries Research Board of Canada 1972;29(10):1463-1466
https://doi.org/10.1139/f72-225
.
64. Sogbanmu TO, Ogunkoya OA, Olaniran EI, Lasisi AK, Seiler TB. Adverse impacts of human activities on aquatic ecosystems: investigating the environmental sustainability perception of stakeholders in Lagos and Ogun States, Nigeria. In: Nubi TG, AndersonI , Lawanson T, Oyalowo B, editors. Housing and SDGs in Urban Africa Advances in 21st Century Human Settlements. Springer; Singapore: 2021. p. 125-145
https://doi.org/10.1007/978-981-33-4424-2_7
.
Figure 1Genotoxic biomarkers of erythrocytes of exposed O. niloticus to subacute concentrations of paraquat (1A) and glyphosate (1B) over a period of 28 days. Key: n=3000; dissimilar letters across columns represents significant differences between the treatment means at p<0.05. Values are presented as mean±SD. 
Figure 2Biochemical biomarkers of the liver of exposed O. niloticus to subacute concentrations of paraquat Figure 2A–E and glyphosate Figure 2F–J over a period of 28 days. Key: GSH-Reduced glutathione, SOD-Superoxide dismutase, GST-Glutathione-S-Transferase, CAT-Catalase, MDA-Malondialdehyde; n=3; dissimilar letters across columns signifies significant differences between the treatment means at p<0.05. Values are presented as mean±SD. 
Figure 3Photomicrographs of longitudinal sections through the gills of Oreochromis niloticus after 14-and 28-days exposure to sub-lethal concentrations of glyphosate, paraquat and untreated. Key: (a) Untreated (day 14)-normal secondary lamellae SL and primary lamellae PL (mag.-× 100); (b) Paraquat (day 14) 0.11 mg/L- mild shortening of the lamellae LN (mag.-× 100); (c) Paraquat (day 14) 1.12 mg/L-severe shortening of the lamellae LN (mag.-× 100); (d) Glyphosate (day 14) 0.01 mg/L-normal gills with PL and SL. (mag.-× 100); (e) Glyphosate (day 14) 0.12 mg/L-mild shortening of the lamellae LN (mag.-× 100); (f) Untreated (day 28)-normal appearing SL and PL (mag.-× 100); (g) Paraquat (day 28) 0.11 mg/L- mild shortening of the lamellae LN (mag.-× 100); (h) Paraquat (day 28) 1.12 mg/L- severe shortening of the lamellae LN (mag.-× 100); (i) Glyphosate (day 28) 0.01 mg/L severe shortening of the lamellae LN (mag.-× 100); (j) Glyphosate (day 28) 0.12 mg/L- mild shortening of the lamellae LN (mag.-× 100). 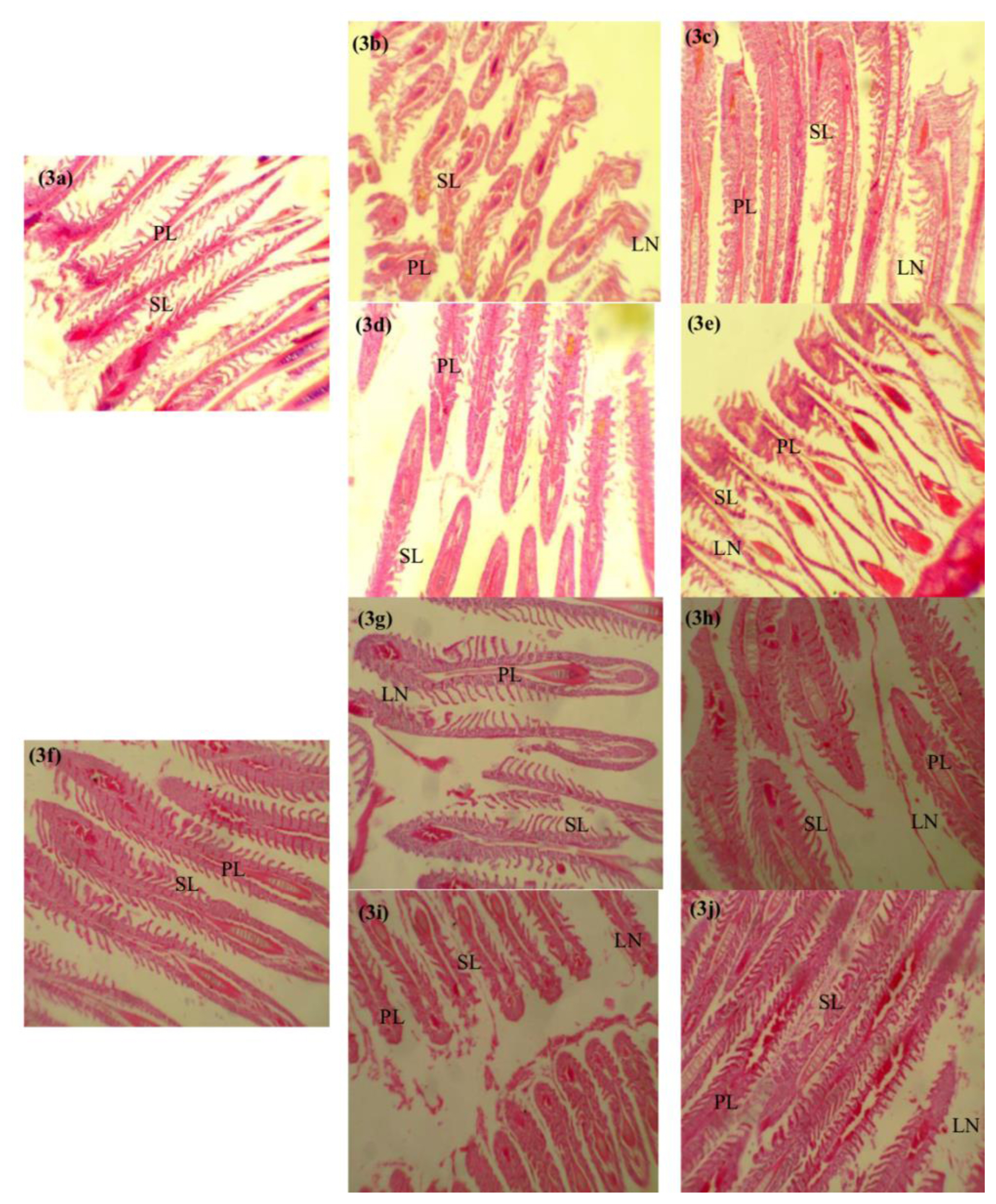
Figure 4Photomicrographs of longitudinal sections through the livers of O. niloticus after 14-and 28-days exposure to sub-lethal concentrations of glyphosate, paraquat and untreated. Key: (a) Untreated (day 14)-Blood sinusoids (BS), central vein (CV), portal vein (PV) and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells, normal liver. (mag.-×100); (b) Paraquat (day 14) 0.11 mg/L-normal liver cells (mag.-× 100); (c) Paraquat (day 14) 1.12 mg/L-normal liver cells (mag.-×100); (d) Glyphosate (day 14) 0.01 mg/L-normal liver cells (mag.-× 100); (e) Glyphosate (day 14) 0.12 mg/L PV and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells with aggregates of inflammatory cells, portal inflammation (mag.- ×100); (f) Untreated (day 28) - BS, CV, PV and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells, normal liver. (mag.-×100); (g) Paraquat (day 28) 0.11 mg/L-portal inflammation cells (mag.-×100); (h) Paraquat (day 28) 1.12 mg/L-portal inflammation cells (mag.-× 100); (i) Glyphosate (day 28) 0.01 mg/L portal inflammation cells (mag.-×100); (j) Glyphosate (day 28) 0.12 mg/L-portal inflammation cells (mag.- ×100). 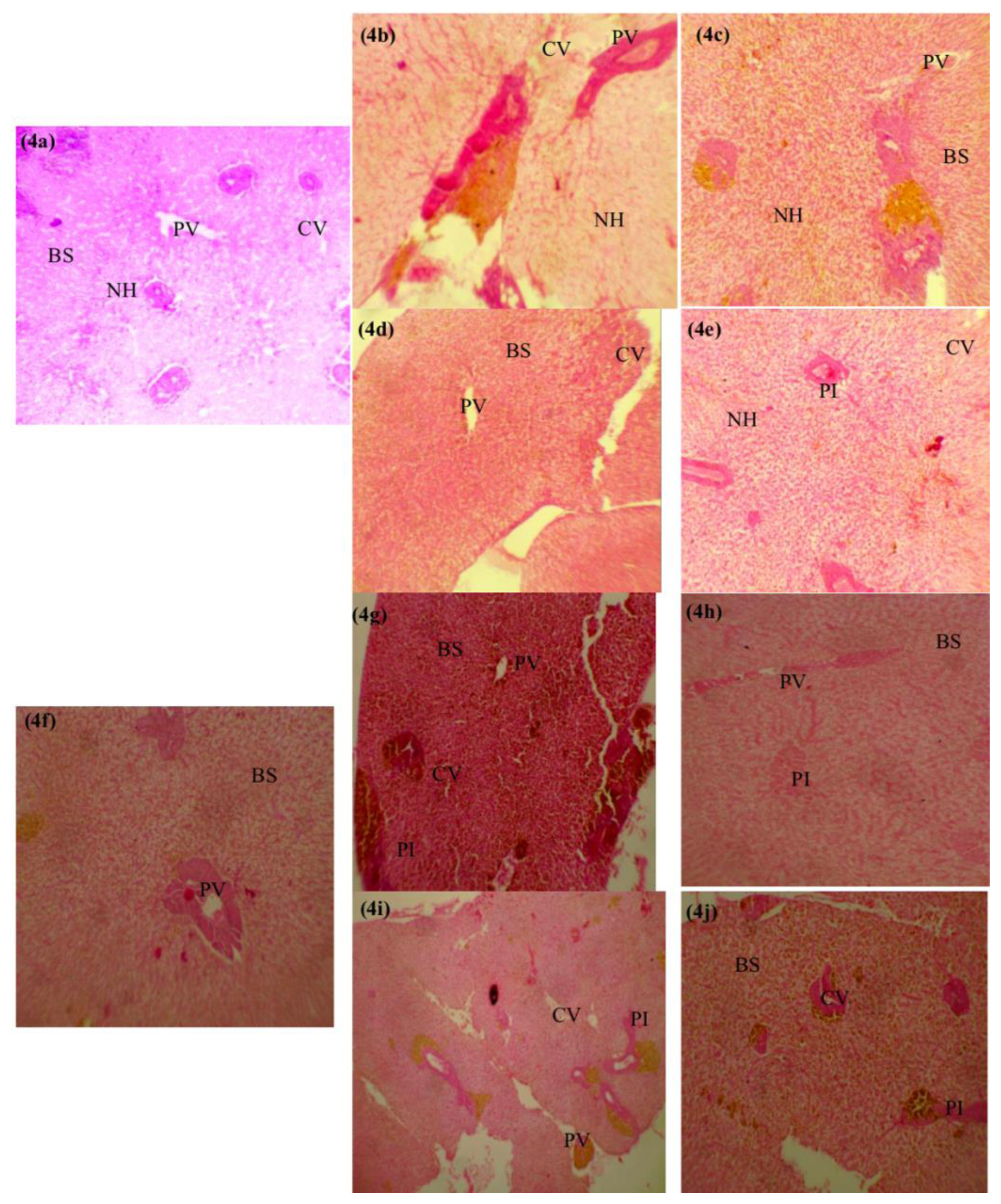
|
|
|||||||||||||||||||||||||||||||||||||||