Kim, Park, Im, Seo, Park, and Nah: Repeated-dose 28-day dermal toxicity study of TiO2 catalyst (GST) in Sprague-Dawley rats
Abstract
TiO2 have been studied on inhalation and skin exposure due to the properties of the materials’ use (cosmetics, paints and other products) and the additional safety information on other intake routes for the potential risk assessment is limited. The aim of this study was to obtain dose-range for subchronic study (repeated 90-day dermal toxicity) new TiO2 powder, GST produced through sludge recycling of the sewage treatment plant through repeated-dose toxicity in Sprague-Dawley (SD) rats. Three test groups for the GST were administered at 500, 1000, 2000 mg/kg B.W/day in addition to a control group (distilled water for injection). 5 male and 5 female rats were included in each group, and we examined the clinical signs, body weights, food consumption, necropsy (organ weights, macroscopic findings), hematological/biochemical parameters and histopathological findings (eye, skin). As a result of observations, there were no treatment-related effects including clinical signs, mortality, necropsy findings etc. Therefore, the present results suggest that the TiO2-related effects were not observed for dermal during 28-day and dose selection for repeated 90-day study was considered to be 500, 1000 and 2000 mg/kg B.W/day under the present study conditions.
Keywords: TiO2, repeated-dose toxicity, NOAEL, GST
Introduction
Currently, TiO 2 (Titanium Dioxide) one of the most frequently used nanomaterials and has been used commercially in cosmetics and skin care products, paints, plastics, paper, toothpicks and other prod [ 1]. Nanoscale TiO 2 such as photocatalyst represents less than 2% of total consumption and presents physical properties and TiO 2 photocatalyst has been used in field of dye-sensitized solar cells and UV protection agents [ 2]. One of the main differences between TiO 2 nanoparticles (NPs) and conventional TiO 2 is the much greater surface area of a given mass or volume of nanoparticles compared to an equivalent mass or volume of conventional TiO 2 particles. This greater relative surface area of the TiO 2 NPs affords a greater potential for properties such as catalytic activity and UV absorption at certain wavelengths. Such properties have led to the development or use of TiO 2 NPs for a wide variety of applications, and also due to changes of dimension, TiO 2 NPs may show different biological, chemical, optical, magnetic and structural properties and may induce differential toxicity [ 3]. Recently, the commercialization of TiO 2 has caused an increase of exposure to human and four main routes of exposure are known for oral/dermal exposures, pulmonary absorption and injection are known for one of the most common forms of route to human and it may translate to systemic organs from the lung and gastrointestinal tract (GIT) [ 4, 5]. The possible biological and safety effects of TiO 2 NPs for dermal exposure and absorption have not been well studied and more investigations on the potential health hazards of the TiO 2 nanoparticles are needed [ 6]. The exposure can be incidental or intentional. One of the possible effects of chemical substances with human exposures is eye and skin irritation. Especially, the skin is the largest organ of the body and can be an important route for the entry of NPs into mammals [ 7]. TiO 2 material, which is covered in this paper, the new TiO 2 material, GST (100% anatase) prepared from the precipitated sludge using TiCl 4 used as a coagulant to remove total phosphorus in the wastewater was manufactured to have cost-competitive lower than price of commercial material (P-25, Evonik Corp., a flame-made multiphasic TiO 2 nanoparticles containing anatase and rutile) with excellent photocatalytic function [ 8, 9]. We have been studied toxicological test as acute oral/dermal toxicity (TG 402, 423) in female rats by Seol et al. [ 10], eye or skin irritation/corrosion in rabbit (TG 404, 405) by Kim et al. [ 11] and 90-day oral repeated toxicity in rats by Kim et al. Through these studies, we confirmed that GST have no treatment-related effect for oral acute/repeated exposure (90-day, subchronic) or acute dermal and eye or skin. But the study for toxicological information on dermal repeated study (90-day, subchronic) has been not conducted. Therefore, the present study was performed to provide dose-range information of subchronic study (repeated 90-day dermal toxicity) for establishing safe levels and formulating risk assessments for new TiO2 catalyst, GST in rats.
Materials and Methods
Test facility
This study was conducted in compliance with the principles of Good Laboratory Practice (GLP) at KTR (Korea Testing & Research Institute), Hwasun based on the Korea Good Laboratory Practice (KGLP) and OECD “Principle of Good Laboratory Practice, ENV/MC/CHEM (98)17 (as revised in 1997)”. The study protocol was reviewed and approved (IAC2021-2555) by the Institutional Animal Care and Use Committee (IACUC) of KTR Hwasun based on the Animal Protection Act [Enforcement Date: 2020-02-12] [No.16977 (2020-02-11, partial revision)] [ 12] and the Laboratory Animal Act [Enforcement Date: 2019-03-12] [No. 15944 (2018-12-11, partial revision)] [ 13]. The KTR Hwasun has been fully accredited by the association for assessment and accreditation of laboratory animal care (AAALAC).
Animal husbandry and maintenance
For the repeated dose toxicity study (Study No. TNK-2021-000584), 44 Sprague-Dawley rats [(5 weeks-old, Crl;CD(SD), SPF] of each sex were obtained from the ORIENT BIO Inc. (8, Hwaaksan-ro 124, Buk-myeon, Gapyeong-gun, Gyeonggi-do, Republic of Korea) and kept carefully following an acclimation period of 8(male)-9(female) days to ensure their suitability for the study. These animals were kept within a well-ventilated and specific pathogen-free (SPF) facility with conditions set to a temperature of 22±3 °C (measured value: 22.1–23.3 °C), a humidity of 50±20% RH (measured value: 44.5–59.7%) with artificial lighting a 12-h light/12-h dark cycle (08:00–20:00/20:00–08:00) and 10–20 air changes per hr. For study, the healthy animals were used after examining conditions included body weight, clinical signs and then 20 rats/sex were randomly divided into four groups listed in Table 1. Animals were kept in stainless steel wire cages and allowed R/O (reverse osmosis) water via a water bottle and irradiation-sterilized pellet diet (Rodent Diet 20 5053, Labdiet, USA), ad libitum.
Test materials and preparation
The new TiO 2 materials, GST (pale yellow powder, crystalline composition of 100% anatase) was provided by Bentec Frontier Co., Ltd (139, Nanosandan-ro, Nam-myeon, Jangseong-gun, Jeollanam-do, Republic of Korea). The characterization of GST was evaluated as Table 2 [zeta potential, particle size image (SEM), TEM (transmission electron microscopy) image, size distribution).
Test procedure
Properties of GST
The characterization of GST showed in Figures 1, 2 and 3, respectively. The zeta potential is the potential between droplet surface and dispersing liquid medium and can be used to estimate surface charge of the droplets in the dispersion medium. Also, it was known for indicator of the droplet stability, where values more positive than +30 mV and more negative than −30 mV indicate good stability against coalescence [ 14]. The estimated value for GST showed that GST has a negative value (−35.4±5.99 mV) and this value was thought to be good stability and it was thought to be a property to be less agglomeration nature ( Figure 1). The particle size value and distribution (95.8±46.3 nm, 46–270 nm) showed that GST was thought to be have materials of various sizes and was difficult to be classified as a nanomaterial considering the definition of nanomaterials (<100 nm) ( Figures 2 and 3).
Clinical signs, body weight and food consumption
All animals were observed and recorded daily [treatment period; twice a day (before/after treatment)] throughout the experimental period (28-day treatment) including treatment-related signs (clinical signs, toxicological symptom and mortality). In the food consumptions, the date values were measured per cage (g/cage/day). Body weight was checked at the time of receipt, assignment, at the start of administration, once a week for administration and necropsy (fasting body weight). Food consumption was measured once a week after the initial of administration. After feeding, the feeding amount on the day and the remaining amount on the next day were measured, and the intake amount was calculated from the difference.
Hematology, biochemistry and hormone analysis
All animals were fasted overnight before blood sampling. The blood samples were collected from the abdominal aorta under anesthesia and transferred to the tubes with anti-coagulant; CBC bottle (EDTA 2K, BD, USA) for hematological test using blood analyzer (ADVIA 2101i, Siemens, Germany), multi-channel microplate reader (Synergy HT, BioTek) and vacutainer (9NC Sodium citrate, BD, USA) for coagulation test using blood coagulation analyzer (ACL ELITE PRO, Instrumentation Laboratory, U.S.A.), respectively. And then, the remaining samples were placed in tubes without an anticoagulant for biochemistry (TBA-120FR, TOSHIBA, Japan) and for hormone analysis (Immunite 2000xpi, Siemens, Germany). To get plasma for coagulation examination, blood samples were centrifuged for 10 minutes (3000 rpm, 4 °C) and the sera for biochemistry/hormone analysis were centrifuged in the same way after the tube were kept at room temperature.
Hematology; Red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), Reticulocyte (Retic), platelet count (PLT), total leucocyte counts (WBC), differential count [neutrophils (Neut), lymphocytes (Lymph, monocytes (Mono), eosinophils (Eos) and basophils (Baso)] Coagulation test: prothrombiN (PT), activated partial thromboplastin time (APTT) Biochemistry: total protein (TP), albumin (ALB), A/G ratio (Albumin/Globulin), total bilirubin (T-BIL), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl-transpepti dase (GGT), creatinine (CRE), blood urea nitrogen (BUN), Urea, total cholesterol (T-CHO), triglycerides (TG), glucose (GLU), calcium (Ca), inorganic phosphorus (IP), creatine kinase (CK), cholinesterase (CHE), total bile Acid (TBA), sodium (Na), potassium (K) and chloride (Cl)
Necropsy and histopathology
On the day scheduled for necropsy, all animals were anaesthetized with anesthetic drug (Ifran Liquid for Inhalation, Hana Pharm. Co., Ltd., Republic of Korea) and device (MatrxTM VIP 3000, MIDMARK, USA) followed by blood sampling. And then, gross examinations were performed on external surface, all internal organs of the cranial, thoracic, and abdominal cavities. Absolute and relative weights (organ weight to fasted body weight ratio, %) of organs were measured for including organs; liver, thymus, brain, pituitary gland, heart, spleen, uterus with cervix, kidneys (*), adrenal glands (*), thyroid gland (*), ovaries (*), prostate + seminal vesicles with coagulating glands, testes (*) and epididymides (*). The bilateral organs (*) were measured, respectively and then the measured weights were summed.
The organs were fixed in 10% neutral buffered formalin, bouin’s fixation (for testes/epididymides), davidson’s solution (for eyes with the harderian gland) as following organs; liver, kidney, adrenal glands, heart, lung (with bronchus), trachea, brain (with pituitary gland), spleen, ovary, testis, epididymis, prostate/seminal vesicles, ovary, uterus (vagina), urinary bladder, tongue, (para)thyroid gland, (small/large) intestine, pancreas, stomach, sternum/femur, skeletal muscle (sciatic nerve), (submandibular/mesenteric) lymph node, spinal cord skin (with mammary gland). The processing for fixed tissues were conducted in eye and skin through trimming, embedding in paraffin, section, and stained with hematoxylin & eosin (H&E). The slide specimens for eye and skin were microscopically examined using the image analyzing system (Leica, Germany) in high dose and control dose group.
Statistical analysis
Data were presented as means ± standard deviation (S.D.) for each group. The body weight, food consumption, organ weights and hematological/biochemical data were analyzed using SPSS (Ver 19.0, Chicago, IL, USA) program. In treatment group, the Leven’s test was performed to derive the homogeneity of variances and one way ANOVA test was performed to determine the significant differences between study groups. In the case of confirming significant difference, post-hoc test was proceeded according to the result of homogeneity (homogeneity; Scheffe tset, heterogeneity; Dunnett’s T3 test). In recovery group, data were performed using independent t-test. P value <0.05 were considered statistically significant.
Results and Discussion
Clinical signs
During the experimental period, there were no abnormal clinical signs and mortality such as treatment-related moribund and dead animals in any of the treatment groups.
Body weight and food consumptions
For the experimental period (28-day treatment period), the body weight showed a normal increase and there were no treatment-related effects in food consumptions between the treatment and recovery group Figure 5.
Hematology and biochemistry
There were no treatment-related significant effects in both sexes in any of treatment group as Tables 3 and 4.
Necropsy and organ weight
At the end of treatment, in gross necropsy, there were no abnormal opinions in both external and internal observation in all administration group of both sexes. Also, as a result of measuring absolute or relative organ weight, there were no significant differences in male and female administration group compared to vehicle control.
Histopathological examination
The histopathological examination was performed to identify the treatment-related effects for treatment group.
Considering retinopathy finding (presence of nanomaterials) observed in previous research of ZnO (particle size: 20 nm, charge: negative) for 90-day in rats [ 15], we examined the eyes carefully including harderian gland and there were no TiO 2-related effects as Figure 5. Also, skin exposed with TiO 2, there were no treatment-related changes considered to be toxicological lesion occurred by test substance administration as Figure 6.
Conclusions
This study was performed to investigate dose-range for subchronic study (90-day repeated dermal study) and evaluate the systemic toxicity for the test substance, new TiO2 (GST), manufactured from sludge recycling of the sewage treatment plant, on male and female Sprague-Dawley rats. 28 days repeated oral dosing via gavage was conducted at dose levels of 0 (vehicle control), 500 (low dose group), 1000 (middle dose group), and 2000 (high dose group) mg/kg B.W/day.
There were no treatment-related effects in body weight, food consumption, hematological examination, biochemical examination, organ weight, gross necropsy and histopathological examination (eye, skin).
In conclusion, the effect related with GST was not observed on male and female Sprague-Dawley rats followed by 28 days of administration period at dose levels of 0, 500, 1000, and 2000 mg/kg body weight. Therefore, dose selection for repeated 90-day study was considered to be 500, 1000 and 2000 mg/kg B.W/day under the present study conditions.
Acknowledgement
This work was supported by a grant (19SCIP-B145906-02) from the Korea Agency for Infrastructure Technology Advancement (KAIA) by Ministry of Land, Infrastructure and Transport of Korea government (MOLIT), Republic of Korea.~
Conflict of interest
The authors declare that they have no conflict of interest.
Notes
CRediT author statement
JHK: Conceptualization, Methodology, Writing-Original draft preparation, MKP: Supervision, Writing-Reviewing and Editing, JMI: Visualization, HSS: Visualization, HJP: Resources, SSN: Project administration, Writing-Reviewing and Editing
References
2. Warheit DB, Brown SC. What is the impact of surface modifications and particle size on commercial titanium dioxide particle samples? - A review of in vivo pulmonary and oral toxicity studies. Toxicol Lett 2019;302: 42-59
https://doi.org/10.1016/j.toxlet.2018.11.008
.   4. Shakeel M, Jabeen F, Shabbir S, Asghar MS, Khan MS, Chaudhry AS. Toxicity of nano-titanium dioxide (TiO 2-NP) through various routes of exposure: a review. Biol Trace Elem Res 2016;172(1):1-36
https://doi.org/10.1007/s12011-015-0550-x
.    6. Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnology, science and applications 2011;4: 95-112
https://doi.org/10.2147/NSA.S19419
.  9. Hossain SM, Park MJ, Park HJ, Tijing L, Kim JH, Shon HK. Preparation and characterization of TiO 2 generated from synthetic wastewater using TiCl 4 based coagulation/flocculation aided with Ca(OH) 2
. Journal of environmental management 2019;250: 109521
https://doi.org/10.1016/j.jenvman.2019.109521
.   10. Seol JK, Park M, Im JM, Seo HS, Park HJ, Nah SS. Acute toxicity assessment for TiO 2 photocatalyst (GST) made from wastewater using TiCl 4 in rat. Environmental Analysis, Health and Toxicology; 2021. 36(3):
https://doi.org/10.5620/eaht.2021019
. 11. Kim SH, Park MK, Seol JK, Im JM, Park HS, Seo HS, Nah SS. Evaluation of potential eye or skin irritation/corrosion in rabbit exposed to TiO 2 photocatalyst (GST). Environmental Analysis, Health and Toxicology; 2021. 36(3):
https://doi.org/10.5620/eaht.2021022
. 14. Alexandru Mihai Grumezescu. Lipid Nanocarriers for drug targeting. Chapter 12. Self-nanoemulsifyomg drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs; 2018. 473-508
http://dx.doi.org/10.1016/B978-0-12-813687-4.00012-8
. 15. Kim YH, Kwak KA, Kim TS, Seok JH, Roh HS, Lee JK, Kang JS. Retinopathy induced by zinc oxide nanoparticles in rats assessed by micro-computed tomography and histopathology. Toxicological research 2015;31(2):157-163
https://doi.org/10.5487/TR.2015.31.2.157
.   
Figure 1
Characterization of TiO2 particles (GST) analyzed by Korea TECH: (a) negative zeta potential (−35.4±5.99 mV, 30 mg/mL); (b) size distribution by intensity (mean: 336.8 nm). 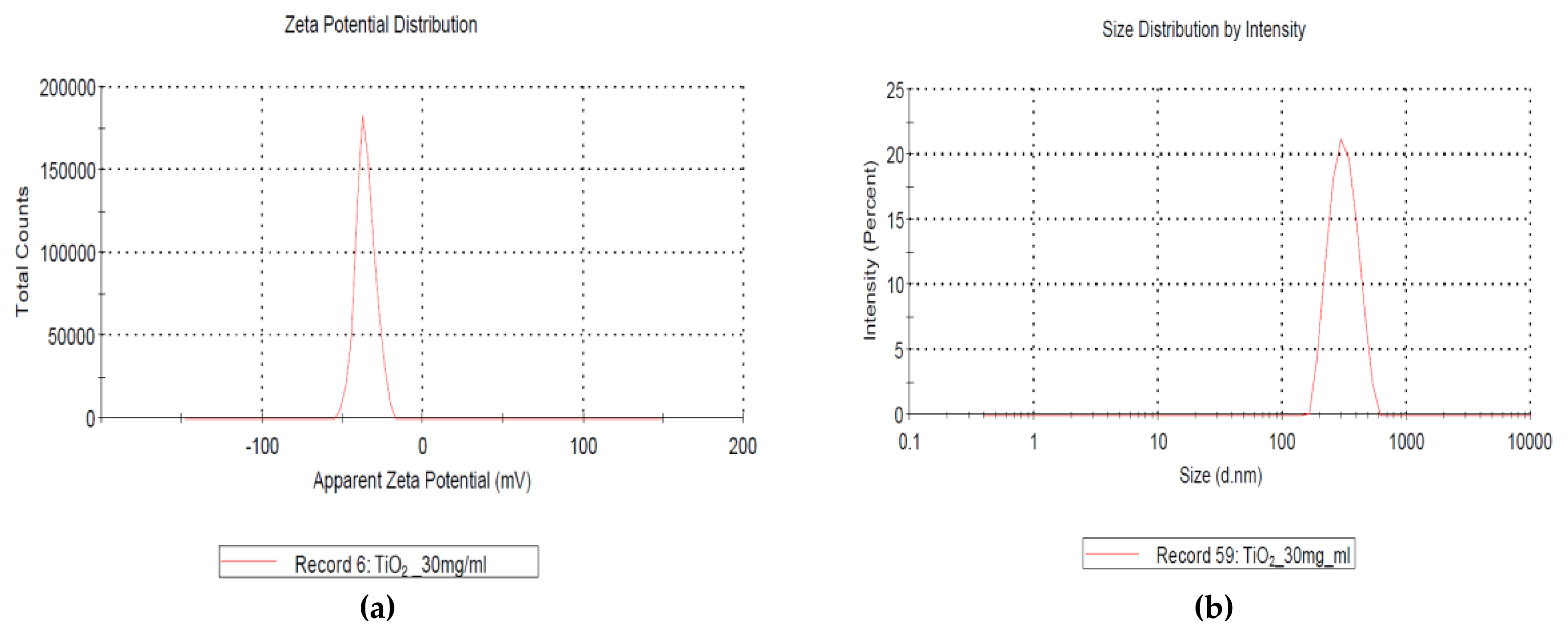
Figure 2
Characterization of TiO2 particles (GST): (a) SEM (scanning electron microscope) image analyzed by KRICT. 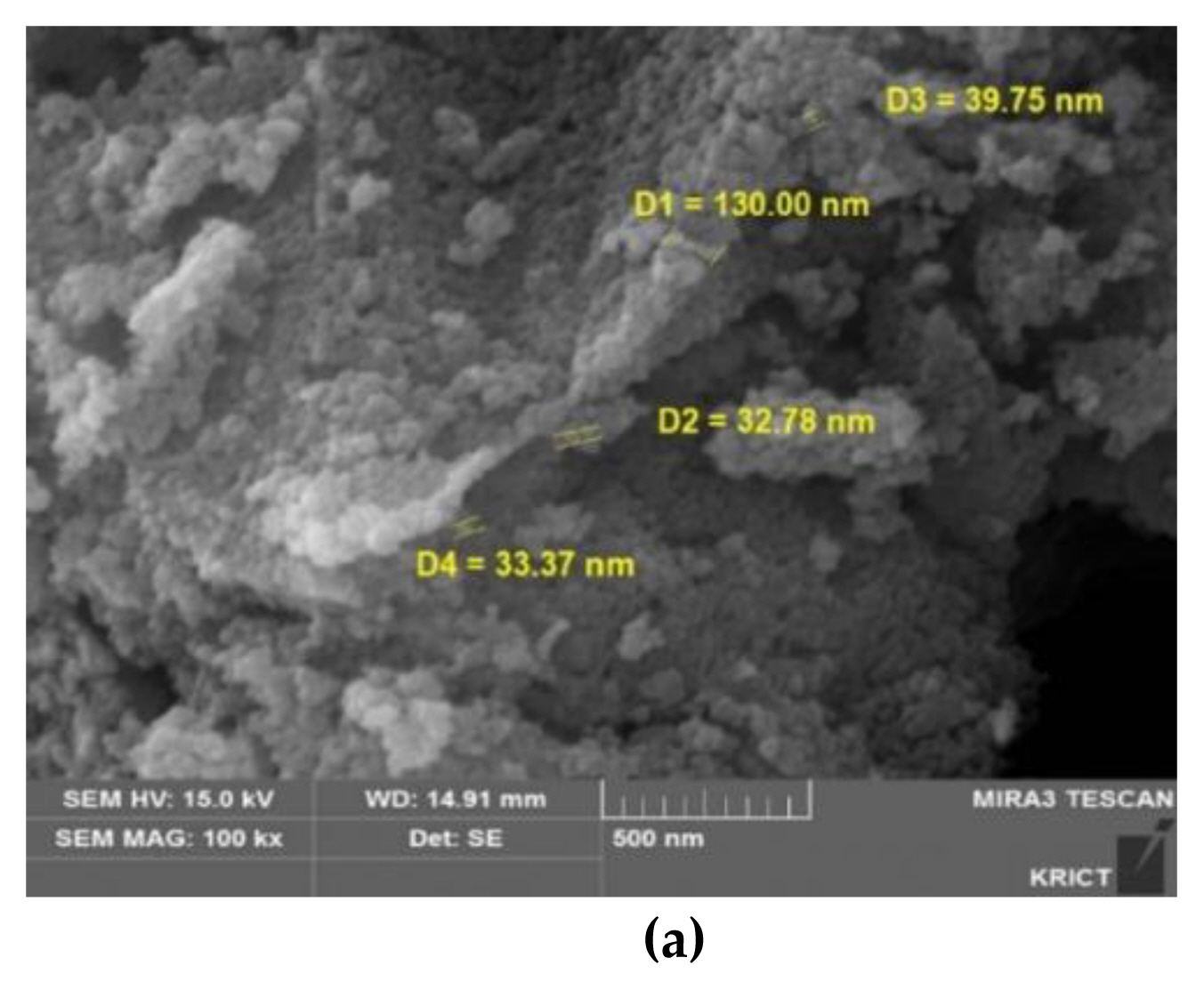
Figure 3
Characterization of TiO2 particles (GST): (a) A particles dispersed in 99.9% EtOH was deposited on a copper grid and analyzed using TEM (Transmission electron microscope) image by Korea Basic Science Institute; (b) Size distribution (95.8±46.3 nm, 46–270 nm,) of the imaged GST(image J software). 
Figure 4
Changes for body weight and food consumptions: (a) body weight (male); (b) Body weight (female); (c) Food consumptions (male); (d) food consumptions (female). 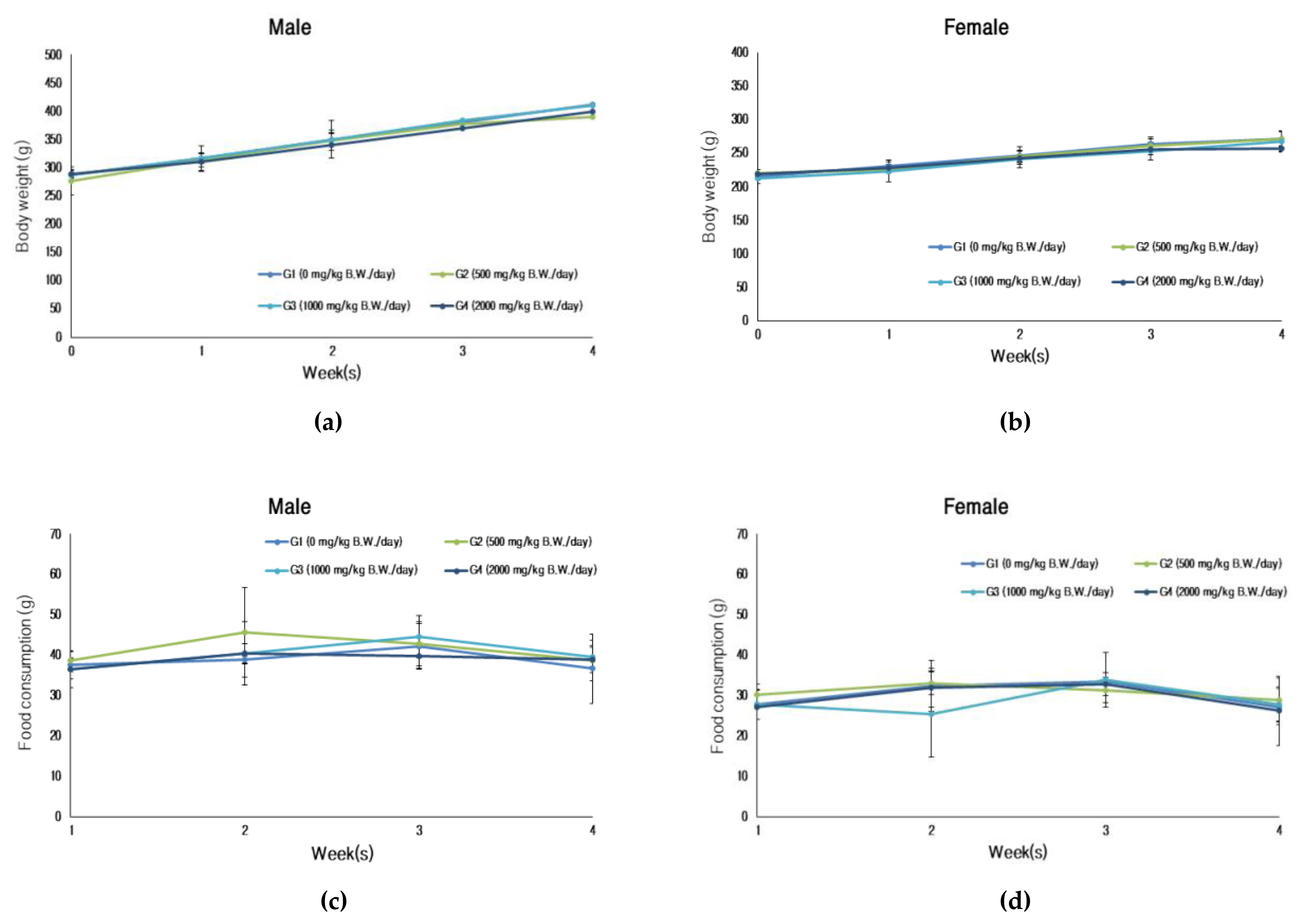
Figure 5
Eye photographs (Left: Slide scanner image, ZEISS Axio Scan. Z1, right: Optical microscope, X50) including the cornea, iris, lens, retina and accessory organs (optic nerve, harderian gland): (a) Control group (animal number 2101); (b) High dose group (animal number 2405). 
Figure 6
Skin photographs (Left: Slide scanner image, ZEISS Axio Scan. Z1, right: Optical microscope, X50) including the epidermis and dermis: (a) Control group (animal number 2101); (b) High dose group (animal number 2405). 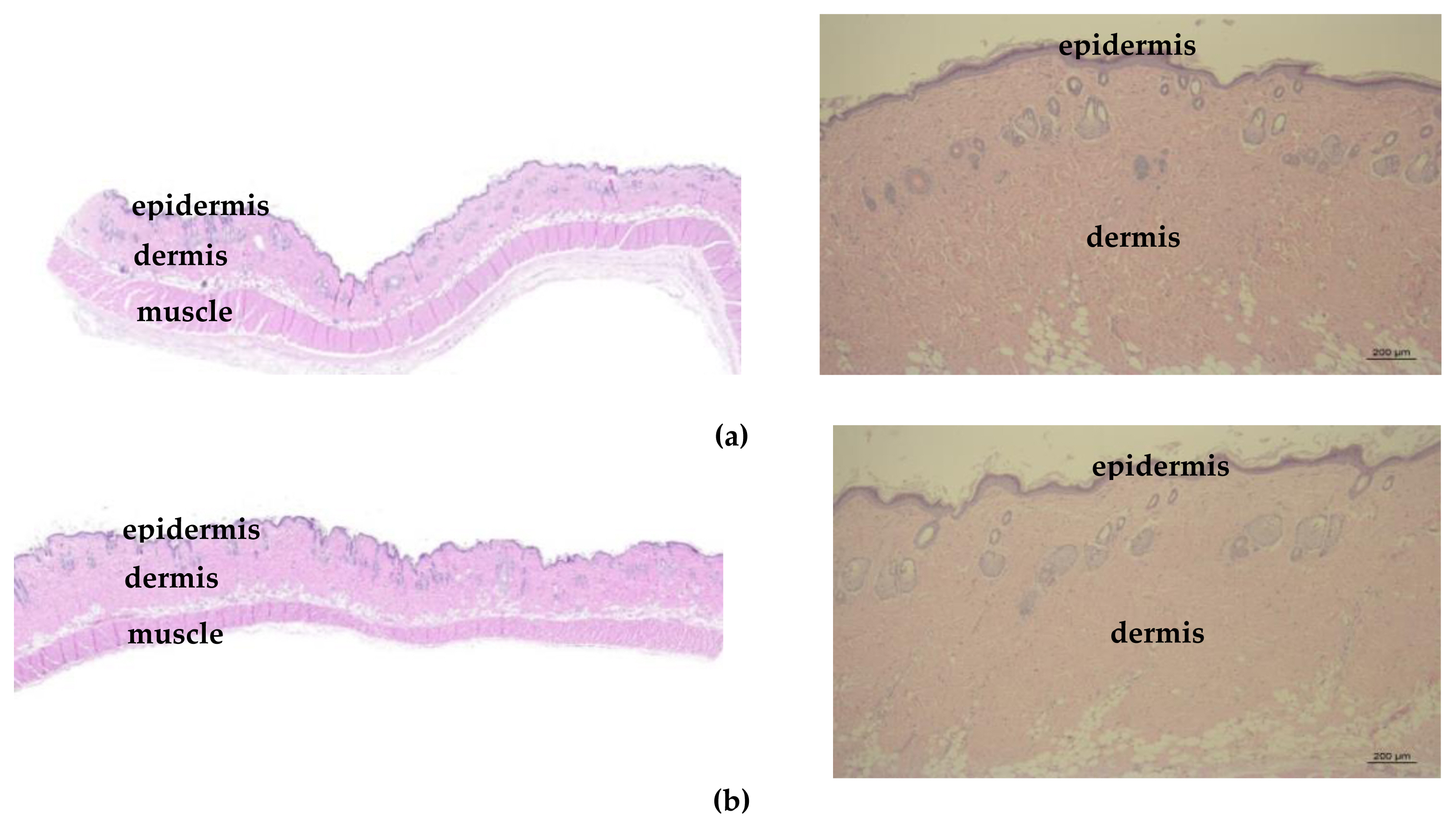
Table 1
Groups for 90-day repeated oral toxicity study.
|
Group |
Dose (mg/kg B.W/day) |
Fluid volume (mL/kg) |
Number animals |
|
(Male) |
(Female) |
|
G1 |
0 |
5 |
5 (1101–1105) |
5 (2101–2105) |
|
G2 |
500 |
5 |
5 (1201–1205) |
5 (2201–2205) |
|
G3 |
1000 |
5 |
5 (1301–1305) |
5 (2301–2305) |
|
G4 |
2000 |
5 |
5 (1401–1405) |
5 (2401–2405) |
Table 2
Evaluation for characterization of TiO2.
|
Item |
Analyzer |
Facility |
|
Zeta potential |
Particle size & Zeta potential analyzer (Zetasizer Nano ZSP, Malvern Instruments LTD., UK) |
Korea TECH*1
|
|
particle size image |
FE-SEM(Field Emission Scanning Electrong Microscope, Tescan Corp., Czech)] equipped with EDS systems (Thermo scientific, USA) |
KRICT*2
|
|
TEM image |
FE-EF-TEM (Field Emission Energy Filtered Transmission Electron Microscopy, JEOL, Japan) |
Korea Basic Science Institute*3
|
|
Size distribution |
Image J software |
- |
Table 3
Hematological parameters.
|
Parameters |
Group(Dose)*
|
G1(0) |
G2(500) |
G3(1000) |
G4(2000) |
|
Sex/Week |
4 weeks |
4 weeks |
4 weeks |
4 weeks |
|
Total leucocyte count (103 cells/μL) |
Male |
7.28±4.29 |
7.53±2.01 |
8.38±2.79 |
7.19±1.62 |
|
Female |
5.28±1.36 |
6.09±1.84 |
4.97±0.58 |
5.09±1.59 |
|
Total erythrocyte count (106 cells/μL) |
Male |
8.18±0.34 |
8.34±0.38 |
8.25±0.47 |
8.22±0.24 |
|
Female |
7.83±0.22 |
8.35±0.33 |
7.98±0.31 |
7.76±0.32 |
|
Hemoglobin concentration (g/dL) |
Male |
15.3±0.3 |
15.5±0.5 |
15.4±0.3 |
15.0±0.2 |
|
Female |
14.5±0.6 |
15.3±0.5 |
14.8±0.5 |
14.4±0.5 |
|
Hematocrit (%) |
Male |
48.0±0.9 |
49.2±1.8 |
48.8±1.3 |
47.8±0.7 |
|
Female |
45.3±1.3 |
47.8±1.5 |
46.0±1.9 |
45.1±1.6 |
|
Mean corpuscular volume (fL) |
Male |
58.8±1.8 |
59.0±1.0 |
59.3±2.6 |
55.1±1.3 |
|
Female |
57.9±1.0 |
57.3±1.2 |
57.6±0.4 |
58.0±1.4 |
|
Mean corpuscular hemoglobin (pg) |
Male |
18.7±0.7 |
18.6±0.5 |
18.7±0.7 |
18.2±0.4 |
|
Female |
18.5±0.5 |
18.3±0.3 |
18.6±0.2 |
18.6±0.5 |
|
Mean corpuscular hemoglobin concentration (g/dL) |
Male |
31.7±0.2 |
31.6±0.4 |
31.6±0.4 |
31.4±0.2 |
|
Female |
32.0±0.7 |
32.0±0.2 |
32.3±0.5 |
32.0±0.2 |
|
Reticulocyte (109 cells/μL) |
Male |
173.9±33.5 |
188.5±39.2 |
195.6±32.3 |
178.3±49.5 |
|
Female |
204.4±79.4 |
190.6±45.0 |
165.0±35.2 |
171.3±46.0 |
|
Reticulocyte (%) |
Male |
2.12±0.38 |
2.26±0.44 |
2.38±0.45 |
2.18±0.64 |
|
Female |
2.62±1.07 |
2.29±0.60 |
2.08±0.49 |
2.23±0.69 |
|
Platelet (103 cells/μL) |
Male |
1107±95 |
1158±108 |
1130±87 |
1174±144 |
|
Female |
1297±133 |
1143±247 |
1106±197 |
1110±102 |
|
Prothrombin time (sec) |
Male |
19.1±4.0 |
16.2±0.9 |
19.7±3.3 |
20.3±3.3 |
|
Female |
12.0±0.4 |
12.1±0.9 |
11.7±0.5 |
12.6±0.7 |
|
Activated partial thromboplastin time (sec) |
Male |
18.6±1.1 |
18.1±1.5 |
18.0±2.3 |
18.2±2.1 |
|
Female |
16.1±1.2 |
15.1±2.7 |
16.4±1.3 |
15.5±1.0 |
|
Neutrophils (%) |
Male |
13.9±4.2 |
12.5±4.0 |
12.7±4.0 |
11.8±3.6 |
|
Female |
12.1±6.8 |
11.7±6.3 |
10.2±3.3 |
13.3±5.7 |
|
Lymphocytes (%) |
Male |
81.6±3.3 |
82.7±4.8 |
83.4±4.4 |
84.1±4.7 |
|
Female |
84.2±7.1 |
83.9±6.2 |
85.7±3.5 |
83.1±5.5 |
|
Monocytes (%) |
Male |
1.2±0.2 |
1.8±0.4 |
1.4±0.5 |
1.3±0.7 |
|
Female |
1.4±1.0 |
1.9±1.2 |
1.2±0.8 |
1.0±0.4 |
|
Eosinophils (%) |
Male |
1.4±0.4 |
1.4±0.6 |
1.2±0.7 |
1.4±0.2 |
|
Female |
1.1±0.2 |
1.2±0.2 |
1.4±0.6 |
1.4±0.4 |
|
Basophils (%) |
Male |
0.2±0.0 |
0.1±0.0 |
0.1±0.1 |
0.2±0.1 |
|
Female |
0.1±0.1 |
0.1±0.1 |
0.1±0.1 |
0.1±0.1 |
Table 4
Blood chemical parameters.
|
Parameters |
Group(Dose)*
|
G1(0) |
G2(500) |
G3(1000) |
G4(2000) |
|
Sex/Week |
4 weeks |
4 weeks |
4 weeks |
4 weeks |
|
Total protein (g/dL) |
Male |
5.5±0.3 |
5.5±0.1 |
5.5±0.2 |
5.4±0.2 |
|
Female |
5.9±0.3 |
6.0±0.3 |
5.9±0.3 |
5.7±0.1 |
|
Albumin (g/dL) |
Male |
4.0±0.1 |
4.0±0.1 |
3.9±0.2 |
4.0±0.1 |
|
Female |
4.3±0.4 |
4.4±0.3 |
4.3±0.3 |
4.1±0.1 |
|
A/G ratio |
Male |
2.7±0.3 |
2.6±0.2 |
2.6±0.6 |
2.8±0.3 |
|
Female |
2.8±0.5 |
2.8±0.2 |
2.7±0.4 |
2.7±0.4 |
|
Total bilirubin (mg/dL) |
Male |
0.01±0.01 |
0.02±0.02 |
0.01±0.01 |
0.05±0.05 |
|
Female |
0.04±0.03 |
0.04±0.02 |
0.03±0.03 |
0.03±0.03 |
|
Alkaline phosphatase (U/L) |
Male |
522±105 |
553±103 |
535±97 |
511±120 |
|
Female |
307±52 |
299±74 |
287±79 |
291±67 |
|
Aspartate aminotransferase (U/L) |
Male |
126±8 |
124±14 |
119±7 |
121±10 |
|
Female |
120±54 |
100±14 |
91±15 |
109±19 |
|
Alanine aminotransferase (U/L) |
Male |
31±4 |
30±4 |
32±4 |
37±6 |
|
Female |
39±14 |
34±8 |
30±4 |
26±4 |
|
Creatinine (mg/dL) |
Male |
0.28±0.02 |
0.30±0.02 |
0.31±0.03 |
0.31±0.03 |
|
Female |
0.38±0.01 |
0.34±0.03 |
0.35±0.03 |
0.35±0.04 |
|
Blood urea nitrogen (mg/dL) |
Male |
13.7±1.1 |
13.7±1.6 |
14.3±1.1 |
14.6±1.5 |
|
Female |
16.1±3.3 |
12.7±0.6 |
15.9±2.6 |
15.7±3.1 |
|
Total cholesterol (mg/dL) |
Male |
57±9 |
48±13 |
50±11 |
48±9 |
|
Female |
58±14 |
55±2 |
62±9 |
52±12 |
|
Triglycerides (mg/dL) |
Male |
18±4 |
27±6 |
23±12 |
16±6 |
|
Female |
10± |
10±2 |
11±1 |
11±7 |
|
Glucose (mg/dL) |
Male |
157±9 |
132±4 |
167±37 |
147±13 |
|
Female |
152±16 |
155±6 |
153±16 |
149±30 |
|
Calcium (mg/dL) |
Male |
9.0±0.2 |
9.2±0.3 |
9.4±0.2 |
9.3±0.3 |
|
Female |
9.5±0.5 |
9.6±.4 |
9.6±0.5 |
9.4±0.3 |
|
Inorganic phosphorus (mg/dL) |
Male |
8.0±0.4 |
7.9±0.3 |
8.0±0.4 |
7.9±0.5 |
|
Female |
7.6±0.4 |
7.2±0.3 |
7.4±0.4 |
7.3±0.7 |
|
γ-Glutamyl transpeptidase (IU/L) |
Male |
1.47±1.01 |
2.36±1.42 |
1.87±1.49 |
2.10±2.39 |
|
Female |
1.79±0.82 |
1.49±0.88 |
0.83±0.36 |
1.33±0.43 |
|
Creatine kinase (U/L) |
Male |
694±185 |
698±144 |
577±138 |
526±94 |
|
Female |
570±781 |
310±100 |
336±219 |
376±300 |
|
Bile acid (μmol/L) |
Male |
15.9±6.9 |
25.7±8.9 |
21.3±13.7 |
15.0±6.9 |
|
Female |
20.5±5.9 |
25.3±7.0 |
19.3±12.7 |
12.3±5.1 |
|
Sodium (mmol/L) |
Male |
146.6±0.7 |
147.1±0.9 |
146.0±1.1 |
146.1±0.6 |
|
Female |
145.2±1.1 |
144.9±0.4 |
144.6±0.7 |
145.5±1.3 |
|
Potassium (mmol/L) |
Male |
4.82±0.10 |
4.98±0.15 |
4.95±0.20 |
4.93±0.17 |
|
Female |
4.64±0.21 |
4.59±0.26 |
4.65±0.27 |
4.44±0.40 |
|
Chloride (mmol/L) |
Male |
101.0±1.0 |
102.1±1.3 |
100.8±1.4 |
102.1±0.7 |
|
Female |
102.8±1.6 |
101.8±1.1 |
101.6±0.6 |
102.9 ±1.5 |
|
Cholinesterase (U/L) |
Male |
4.2±.0.8 |
4.4±0.8 |
3.7±0.8 |
3.9±0.5 |
|
Female |
3.8±2.5 |
3.6 ±1.4 |
3.6±1.3 |
4.3±1.7 |
|
|


















