Introduction
The unique properties of the engineered nanomaterials (ENMs) that make them attractive for a plethora of applications including their use in microelectronics, catalysts, composite materials, and biotechnologies. Since the ENMS are incorporated into a growing variety of products ranging from common household items to novel medical technologies, it also invokes concerns for the equally unique human and environmental risks associated with the use of these materials [1,2]. Despite the mechanistic understanding for the many benefits to using the ENMs, their environmental implications are not fully understood. Therefore, it requires novel approaches to access the likelihood of the predicted concentrations of exposure over time.
To predict the concentrations at different points of release (e.g., the effluent of wastewater treatment plants (WTP), the waste of the incineration treatment plants (WIP), and the biosolids left in landfills), it is necessary to estimate the magnitude of release of the ENM to water, soil, and air [3]. To understand the environmental exposure of the ENMs, the global ENM production should be considered. One recent report for global ENM production was based on a market study as well as a newly available European regulatory review of ENMs [2]. In the Future Markets report, the global ENM production as of 2010 was estimated as 268,000 to 318,000 metric tons per year. Except for carbon black, the TiO2 nanomaterial is produced most often; in order of most to least produced ENM, TiO2>SiO2>ZnO>Fe and FeOx>Al2O3>CeO2>CNT>Ag [4]. In South Korea, the usage amount of ENMs was investigated by the South Korea Chemicals Management Association and the National Institute of Environmental Research (NIER) from 2007 to 2012. As with global ENM production reports, the ENMs most used in order from most to least is: SiO2, Al2O3, TiO2, ZnO, CeO2, Ag, and CNT [5]. Among the major countries of the ENMs consumption, China consumes 47% of Asia’s ENM. The UK accounts for 9% of Europe’s ENM use, and the US accounts for 74% of North American use of the ENMs [2].
As mentioned above, a plethora of applications of the ENMs caused intentional or unintentional exposure of the ENMs to human and environmental media. Therefore, the ENMs can be added to soils directly in fertilizers or plant protection products or indirectly through application to the land or wastewater treatment products such as sludges or biosolids. Additionally, the ENMs may enter aquatic systems directly through industrial discharges or from the disposal of WTP effluents [6]. They may also enter aquatic systems indirectly through the surface runoff from soils.
Although a study on the environmental exposure of the ENMs is essentially required, few reports exist as compared to the studies on the novel synthesis and cytotoxicity of the ENMs. As shown in Figure 1, the percentage of publications for the synthesis and cytotoxicity of silver nanoparticles (AgNPs) is over 60% and 15%, respectively. However, the publications for other keywords, such as fate, wastewater treatment, sludge, incineration, etc., were few in number. Therefore, to prevent the unintentional release to the environmental media (air, water, and soil) and to reduce the human and environmental toxicity of the ENMs, in-depth research on the environmental exposure of the ENMs and its wastes (i.e., nanowastes containing ENMs) is needed.
Herein, a recent research trend for the exposure of the ENMs in environmental media was reviewed. First, the environmental concentrations of the ENMs should be required to predict the quantity of the emission of the ENMs in the environmental media. Then, the major exposure sources of the ENMs can be revealed based on the predicted environmental concentrations (PEC) of the ENMs. Second, to better understand the unintentional release of the ENMs, the treatment (or removal) efficiency in the nanowastes treatment facilities (WTP and WIP) should be investigated. Landfills of nanowastes should also be investigated.
Modeling Studies on Predicted Environmental Concentrations of Engineered Nanomaterials
The challenge in assessing the PEC is that there are no standardized measuring methods for the nanoparticles in the environment even to this day. Furthermore, no regulation and duty of declaration for the nanoparticles exist, and the production volumes of most companies are confidential. Also, investigating the predicted no effect concentration (PNEC) of the nanoparticles is challenging because their toxicity depends on many factors such as the surface coatings, and size of the nanoparticles [7]. Generally, the prediction of ENM levels in environment is based on the estimated worldwide production volume and a substance flow analysis from the ENM-containing products to the three environmental compartments or media: air, soil, and water. As recently summarized [8], the work by Swiss Federal Laboratories for Materials Science and Technology group has led the way in developing methods for estimating the ENM production and emissions. Several researchers have tried to combine analytical techniques and modeling data to get the first quantitative information on their occurrence in technical and natural systems [8-11]. However, there is uncertainty or distinct lack of knowledge about the most influential parameters: the potential production, application, and environmental release volumes; the physicochemical contaminant properties; the background concentrations; and the environmental fate/behavior of these materials. Therefore, it is not easy to guarantee the accuracy oft the PEC obtained by modeling.
Modeling the ENM in the environment began with Boxall et al. [9], who presented the first quantitative approach for assessing the ENM release and concentrations for the environmental media. Mueller & Nowack [10] went one step further and, for the first time, used a material flow analysis (MFA) to replace the hypothetical calculations. Using a life-cycle perspective, this MFA combined assumptions and the initial empirical information on the ENM production quantities, release rates, and behavior in the technical compartments. Gottschalk et al. [11] used a probabilistic material flow analysis (PMFA) approach that builds on the Monte Carlo (MC) computer simulations for predicting the PECs of five ENMs (TiO2, ZnO, Ag, CNT, and fullerenes) in the water, sediments, biosolids, soils, and air. Therefore, the PECs provided by this model were the most up-to-date, comprehensive values at that time. Since several studies have been more limited in scope in terms of spatial scale, life cycle, applications, or a range of ENMs, Lazareva & Keller [2] provided the first view of the global mass flow of ENMs using the global production estimates. They covered of a wide range of applications and types of the ENMs. They also covered the country-specific data sets for the economic development, the handling of wastewater, the incineration of biosolids, and the methodology to convert the information to a local level.
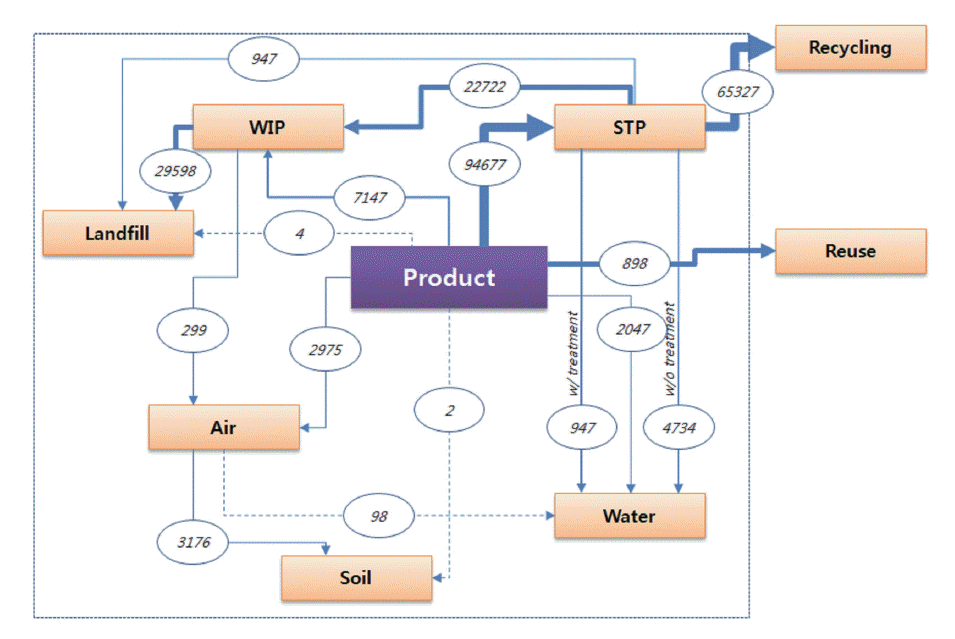
The PEC values are available for the surface water, WTP effluents, biosolids, sediments, soils, and air, and this data collection could be extended to a preliminary assessment of the ENMs in environmental media. Based on the inventory data for usage of the ENMs in South Korea [5], the PEC values could be obtained based on the modified Mueller box-model as shown in Figure 2 and Table 1. In the latest papers for the PEC modeling [2,7-11] and the NIER report [12], the major routes for the environmental exposure of the ENMs were revealed as the WTP effluents/sludge. For example, in the case of nano-TiO2, the PEC value in the air, surface water, WTP effluent, and biosolid was calculated as 1.5×10-6, 0.7, 180, 0.6 μg/L, respectively [10]. Therefore, to assess the environmental fate of the ENMs in the environmental media, the treatment efficiency of the nanowastes in the WTP and other facilities should be investigated.
Nanowastes Treatment in Wastewater Treatment Plants
According to the nanotechnology consumer products inventory by the Project on Emerging Nanotechnologies, of the 1,628 nanotechnology-based consumer products available on the market, the products containing AgNPs and TiO2 accounted for the largest (383 and 179 products, respectively) and fastest growing category [13]. Therefore, the intentional or unintentional release of pristine and aged ENMs to the environment is largely unavoidable. For example, TiO2 and ZnO can be released from the consumer products (sunscreens and UV-absorber lotion) to the WTP through washing [14,15]. As is the case for most other the ENPs like TiO2, the majority of the ENMs in consumer products will be likely released into the sewer systems. Therefore, the municipal WTP act as the gateways that control the release of the ENMs from domestic and/or industrial sources to the aquatic environment via treated effluent that is discharged into surface waters [16].
Based on batch bioreactors in a laboratory, previous investigations on ENMs (especially AgNPs) removal have shown that about 90% spiked ENMs are efficiently reduced by biological treatment and accumulated in the activated sludge or biosolids [17]. To confirm the removal efficiency of the ENMs by activated sludge in the real world, further investigation of the ENMs removal using field-collected wastewater influent, semi-treated wastewater (i.e., wastewater treated by mechanical devices like screen and grit chambers before biological treatment), and effluent is necessary.
In a recent model simulation, the concentration of AgNPs is estimated to be 21 ng/L and 1.55 mg/kg in the effluent and sludge of the WTP, respectively [11]. As they have shown strong antimicrobial activities against a variety of microbes, there are increasing concerns about the potential negative impact of AgNPs on the waste and wastewater treatment performance. When TiO2 is released to bodies of water, the estimated concentration ranges from 0.7 to 16 μg/L [10]. Even though low concentrations of released ENMs from the effluent of WTP occur, the continuous exposure of the ENMs to aquatic organisms can cause chronic toxicity.
Several studies on the specific ENMs removal and release from the WTP are reported [14-21]. In analyzing the field-collected samples from nine municipal WTPs in Germany, more than 72% of the remaining AgNPs in wastewater after mechanical treatment were reduced by biological treatment [16]. Together, these processes reduced 95% of AgNPs that entered the WTPs, thereby resulting in low concentration of AgNPs in the effluents (<12 ng/L). A recent study on the exposure of AgNPs in activated sludge treatment system suggests that more than 90% of AgNPs were associated with the biomass and the total silver concentration in the effluent wastewater was below 0.05 mg/L [18]. At one WTP study where raw sewage contained 100-300 ppb Ti, it accumulated in settled solids at concentrations ranging from 1 to 6 μg Ti/mg biosolids [19]. Since the sludge containing ZnO and TiO2 could be finally dumped into landfill sites, it is estimated that about approximately 75% of the total TiO2 entering the WTP would finally end up in landfills. In a model test for the removal of oxide nanoparticles [CeO2, TiO2, SiO2, Fe2O3, ZnO, and Ca3(PO4)2] in the WTP, a majority of oxide nanoparticles could be captured through adhesion to clearing sludge. A significant fraction of the engineered nanoparticles escaped the wastewater plant’s clearing system, and up to 6 wt % of CeO2 was found in the exit stream of the model plant [20]. Therefore, the wastewater effluents are discharged primarily to surface waters and represent a significant potential point source for the nanomaterials enetering into the environment.
While continuous input of AgNPs into the wastewater did not significantly alter chemical oxygen demand (COD) removal, the NH4 removal was reduced at the beginning of the batch experiment [22]. In the near future, it is likely that the surfactantstabilized ENMs released into sewage will cause significant adversary effects on the the COD and NH4 removal of activated sludge processes in the municipal wastewater treatment plants. Therefore, the cytotoxicity of the ENMs in microogranismsiactivated sludge should be studied.
As mentioned above, the ENMs that entered in the WTP were mostly removed and accumulated in the sludge and biosolids. Ultimately, this waste sludge that contained the ENMs and consumer products are placed in landfills at the end of their lifetime. Since the biosolids are usually used as agricultural land amendments (fertilizers), placed in landfills, incinerated, or dumped into oceans, the biosolids is a potential source of ENMs release into the environment. This point source is very different from the WTP effluent discharge, and these biosolid releases and the resulting ecosystem exposures remain poorly understood [19].
Nanowastes Treatment by Waste Incineration Plant
More than 100 million tonnes of municipal solid waste are incinerated worldwide every year. However, little is known about the fate of the nanomaterials during incineration even though the presence of engineered nanoparticles in waste is expected to grow [23]. China plans to expand its capacity for the waste incarnation from 3% in 2011 to 30% by 2020 to minimize the amount of untreated landfill waste [24]. The ENMs were introduced either directly onto the waste before incineration or into the gas stream exiting the furnace of an incinerator, and thus, the waste ash released from the WIP might contain the ENMs. As mentioned earlier about the nanowaste treatment by the WTP, the ENMs from the wastewater was transferred efficiently to the sludge, which is sometimes then incinerated. Because the waste incinerators are complex systems that include interaction effects arising from the heterogeneous waste matrices, the fate and the removal efficiency of the ENMs in the WIP are not yet known.
Recently, one research paper for the persistence of the ENMs in the WIP was published [23]. Walser et al. [23] show that the CeO2 nanoparticles introduced into a full-scale WIP bind loosely to the solid residues from the combustion process and can be efficiently removed from the flue gas using current filter technology. They concluded that it is possible to incinerate waste without releasing the ENMs into the atmosphere. Instead, the residues to which the ENMs bind eventually end up in landfills or recovered raw materials. Namely, they shift the disposal problem to the subsequent processing steps, the landfills, and the final deposits where the slag and fly ash residues are eventually handled and stored.
Additionally, one paper reported on the formation of the polycyclic aromatic hydrocarbons (PAH) and dioxins from the incineration of paper and plastic waste containing the ENMs, including TiO2, NiO, AgNPs, CeO2, and C60 [25]. Depending on the type of waste, the presence of the ENMs in the waste stream resulted in higher emissions of some PAH species. Chlorinated furans were formed at elevated concentrations with wastes containing AgNPs and TiO2. They said that the combination of the high specific surface area and catalytic, including electrocatalytic, properties of nanomaterials might be responsible for affecting the formation of toxic pollutants during incineration.
Although the WTP and WIP are efficiently used for the removal of the ENMs, the waste sludge and slag/ash containing the ENMs should be additionally treated. In other words they should be landfilled as the end-of-life cycle of the ENMs and consumer products.
Nanowastes Treatment by Landfill
It is well known that silver ions and AgNPs in wastewater are mostly accumulated in sludge. Furthermore, AgNPs can be adsorbed on the sludge and embedded in the sludge to form new products such as Ag2S [17]. Ultimately, landfills are the likely final destination of the disposed sludge or discarded ENMs products. In a recent model simulation, an average of 4.77 tons Ag- NPs per year is estimated to be dumped into landfills [8].
Mueller et al. [26] showed the waste disposal as the input-output system for the ENMs based on the box-modeling and scenario analysis. It shows that for TiO2, ZnO, and AgNP, bottom ash to the landfill is expected to cover 58%-62% the most important flow. Also, the direct deposition on landfills, mainly with construction waste, is predicted between 23% and 29%. All of the other flows, e.g. export of fly ash, landfilling of fly ash, etc., are much less important. The release into the water and air are almost nonexistent. For CNT, the flows are very different with about 94% burned and, therefore, destroyed. This result means that the incineration can have a strong influence on some ENM but that the majority of the ENM-mass still is expected to end up in landfills.
Additionally, some toxic ENMs in the landfill might reduce methane production by inhibiting methanogenesis. While silver ions had no impact on the landfill production of methane at the concentration of 10 mg/kg solids, AgNPs at that concentration inhibited methanogenesis. This inhibition might be due to the slow and long-term silver ion release from the AgNPs dissolution in the landfill [27].
Only recently, the disposal of the nanowastes into landfills has raised concerns about the effects of the added ENMs on the waste degradation and leachate treatment associated with landfills in addition to the potential release of the ENMs to the environment through the interaction with landfill leachate [28]. The majority of the ENMs in landfill leachate aggregated and was present as larger particles which might be retained in the solid waste as the leachate moves through the landfill. This result means that the long-term presence of the ENMs in the landfill and landfill leachate could act as continuous releasing source of the ENMs to soil and underground water.
Conclusion
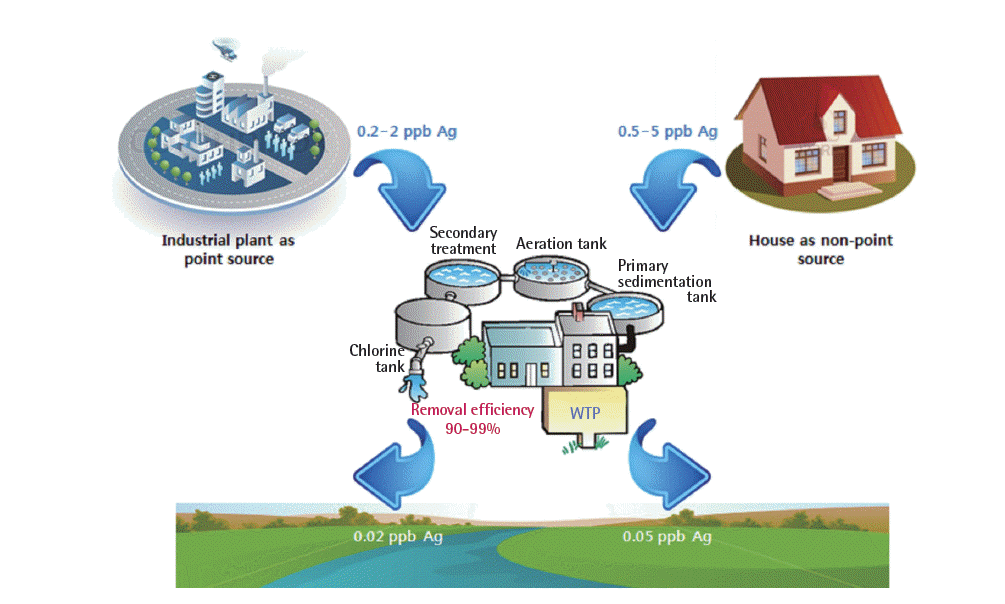
The ENMs are intentionally or unintentionally released to environmental media during the life cycle of consumer products. Recently, to understand the environmental exposure of the ENMs and to reduce the unintentional release into the environment, several model studies were discussed, and the key releasing points were found as the WTP effluent/sludge. The nanowastes that contained the ENMs were treated using WTP and WIP. Reassuringly, the WTP and WIP efficiently removed the ENMs from aqueous phase and emitted clean gas, which poses no risk to the environment (Figure 3). Since these nanowaste treatments are not an effective end-of-life treatment, the ENMs emissions can occur during the further treatment of the waste sludge in the WTP or the slag in the WIP. Therefore, the remediation or recovery of metals from the nanowastes was an additionally required step with so-called landfill mining techniques. Additionally, the monitoring for the ENMs in the environment will increase the information on the fate and transport of the ENMs in the environmental media.