AbstractObjectivesThe sub-acute toxic effects following repetitive intramuscular injection of two cervical cancer vaccines newly developed against human papillomaviruse (HPV)16/58/18 and HPV16 were investigated in female ICR (CrljOri: CD1) mice, and the no-observedadverse- effect-level (NOAEL) of the cervical cancer vaccines was estimated.
MethodsFemale ICR mice (n=15 in each group) were exposed to a 1:1 mixture of two cervical cancer vaccines by repetitive intramuscular injection (once a week, 5 times) for 5 weeks. Mortality, body weight, organ weight, hematological/biochemical parameters, and histopathological effects were examined at different concentrations (0, 1×108, 5×108, and 2.5×109 copies/animal) of the cervical cancer vaccines.
IntroductionCervical cancer shows the seventh highest incidence rate of malignant cancers in Korean women. The sexually transmitted infection of human papillomaviruses (HPVs) has been established as the major causative agent of cervical cancer [1,2]. HPVs have been found in more than 150 viruses [3]. The highrisk types of HPV are 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, 73, and 82, while the low-risk types of HPV are 6, 11, 42, 43, and 44 [4,5]. High-risk HPV types have been found in more than 90% of cervical carcinomas or intraepithelial neoplasia, and, in particular, two HPV types (16 and 18) are responsible for about 70% of cervical cancers [6,7].
Vaccination is the most effective means of preventing HPV infectious diseases [8]. Several HPV vaccines have been developed, and two cervical cancer vaccines (Gardasil® and Cervarix ®) have obtained Food and Drug Administration approval in the USA [9]. Gardasil® for girls and boys is a quadrivalent vaccine against HPV 6/11/16/18 and Cervarix® for girls only is a bivalent vaccine against HPV 16/18 [9]. The distinct efficacy of two vaccines on HPV infectious diseases was confirmed in large randomized clinical trials [10-14]. However, adverse effects of HPV vaccines, such as vasovagal syncope, venous thrombosis, pulmonary embolism, and amyotrophic lateral sclerosis, have been reported in several studies, although it is difficult to infer clear causal associations between HPV vaccines and adverse effects [15,16]. Therefore, it is important to evaluate the safety of newly developed vaccines for cervical cancer.
In this study, we investigated the sub-acute toxic effects in female ICR mice following repetitive intramuscular injection of new cervical cancer vaccines, i.e., Ac-HERV-16-58-18 L1 and Ac-HERV-LANPSS-E6E7, which target HPV 16/58/18 and HPV 16, respectively. The no-observed-adverse-effect-level (NOAEL) was also evaluated.
Materials and MethodsMaterialsTwo vaccines for cervical cancer were manufactured by Kolon Life Science Inc. (Gwacheon, Korea): Ac-HERV-16-58-18 L1 and Ac-HERV-LAMPss-E6E7 were developed against HPV types 16/58/18, and HPV 16, respectively. The vaccines were suspended in Dulbecco’s phosphate-buffered saline (DPBS), and stored at 3.5-6.0°C until administration to mice. A 1:1 mixture of the two vaccines (Ac-HERV-16-58-18 L1 and Ac-HERVLAMPss- E6E7) was injected into female ICR mice.
AnimalsBecause cervical vaccines are administrated in women only, female ICR mice were used to examine the toxic response of cervical vaccines. Specific pathogen-free female ICR mice (5 weeks old, 19.4-23.6 g) were purchased from Orient Bio Inc. (Seongnam, Korea). They were maintained in an environmentally controlled room under standard conditions of temperature of 21.2- 23.9°C, relative humidity of 40.7-70.2%, air ventilation of 10-15 times/hr, and a 12-hour light/dark cycle of 150-300 lux. The animals were fed with standard pellet (Harlan Laboratories, Indianapolis, IN, USA) and filter-sterilized tap-water ad libitum. All animals were housed for a 6-day acclimation period. During this period, each animal was observed once a day for health conditions and body weights were measured. Female mice were distributed into four groups (G1: control group; G2: 1×108 copies/animal; G3: 5×108 copies/animal; G4: 2.5×109 copies/animal) by stratified random sampling based on body weight, with each group containing fifteen mice (Table 1). The animals were ethically handled according to the agreed guidelines for the Biotoxtech Co., Ltd (Cheongju, Korea).
Experimental Design and AdministrationA 1:1 mixture of two vaccines (1×108 copies/animal, 5×108 copies/animal, and 2.5×109 copies/animal) was administered by repetitive intramuscular injection using a 26-gauge needle once a week for 5 weeks. A control group was injected with DPBS only. Ten female mice were euthanized for the assessment of hematology, blood biochemistry, organ weight, and necropsy/histopathological findings. The test was performed following Good Laboratory Practice according to the “Guidelines for Toxicity Tests of Drugs and Related Materials” provided by the Korea Food and Drug Administration.
Organ Weights, Clinical Signs, and MortalityAll animals were observed at least twice daily for any clinical signs of toxicity (behavioral pattern: salivation, fur, lethargy, and sleep, changes in physical appearance, injury, pain, and signs of illness) and mortality by macrograph during the experimental period. All animals were monitored for body weight changes once a week after administration.
Histopathological EffectsAt the conclusion of the 5-week experiment, food was withheld for 4-hour and the mice were anesthetized by isoflurane inhalation. After blood collection, female mice were sacrificed by exsanguination of the abdominal aorta. Brain, pituitary, heart, lung, liver, spleen, kidney, adrenal gland, ovary, and uterus were carefully removed and weighed for absolute/relative organ weights. The organs were fixed in a 10% formalin solution, embedded in paraffin, and stained with hematoxylin and eosin for histopathological examination.
Hematological and Serum Biochemical ParametersPrior to necropsy, blood was drawn from the inferior vena cava using a syringe with a 24-gauge needle. One portion of the blood was treated with ethylenediaminetetraacetic acid and analyzed for total red blood cell count (RBC), hemoglobin concentration (Hgb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT) count, total white blood cell count (WBC), and differential leukocyte percentage (neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), eosinophils (EOS), and basophils (BASO)) using a hematological autoanalyzer (MS9-5; Melet Schloesing Lab., Osny, France). Another blood sample was treated with sodium citrate for blood clotting parameters prothrombin time (PT) and activated partial thromboplastin time (APTT) using an autocoagulation analyzer (Coapresta 2000; Sekisui Medical Co., Tokyo, Japan). Serum from one remaining blood sample was obtained by centrifuging at 3,000 rpm for 10 minutes, and was used for serum biochemical analysis. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose (GLU), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total cholesterol (T-Chol), triglycerides (TG), total protein (TP), albumin (Alb), albumin/ globulin (A/G) ratio, phosphorus (P), and calcium (Ca) were analyzed using a biochemistry autoanalyzer (Hitachi 7060; Hitachi, Tokyo, Japan).
Statistical AnalysisStatistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Data of body weight, organ weight, and hematological and biochemical parameters in female mice exposed to vaccines for 5 weeks were expressed as the mean±standard deviation, and were analyzed for homogeneity using Barlett’s test. Tests of significance between the four groups were performed using Dunnett’s t-test after ANOVA for homogeneous data or using the Kruskal–Wallis test for non-homogeneous data. A p-value of < 0.05 was considered significant.
ResultsHematological and Serum Biochemical ParametersIn the hematological analysis (RBC, Hgb, Hct, MCV, MCH, MCHC, PLT, and WBC), and differential leukocyte counting (NUT, LYMPH, MONO, EOS, BASO), the treated groups showed no significant changes compared to the control groups (Table 2). The blood clotting parameters (PT and APTT) did not show a significant difference between the control and treated groups (Table 2).
As shown in Table 3, serum biochemical analysis (ALT, AST, ALP, GLU, BUN, Crea, T-Bili, T-Chol, TG, TP, Alb, P and Ca) showed no significant difference between the control and treated groups. However, the A/G ratio in the vaccine-treated groups G3 and G4 showed a significant decrease compared to G1.
Histopathological EffectsAs shown in Table 4, repetitive muscular injection of vaccines induced several histopathological signs, including mononuclear cell infiltration and mineralization (myofiber). However, animals in the control group showed no symptoms at the site of injection.
The histopathological effects on major organs (kidney, parathyroid gland, salivary gland, spleen, thyroid, etc.) were investigated in G1 and G4 (Table 4). Several animals showed histopathological effects, including cystic tubules in the kidney, ectopic thymus in the parathyroid gland, cell infiltration in the salivary gland, and extramedullary hematopoiesis in the spleen. However, these effects were similar when comparing the G1 and G4 groups. In histopathological findings, the animal in G1 showed aplasia of the right uterus horn (Table 4).
DiscussionSeveral vaccines targeting high- or low-risk HPV types have been used to protect women from cervical cancer caused by HPV infection. Vaccines have been reported to induce several side effects, including muscle pain or tenderness, seizures, and weakness in clinical trials [9]. Therefore, side effects of newly developed vaccines must be evaluated for safe use of vaccines. We developed two new vaccines (Ac-HERV-16-58-18 L1 and Ac-HERV-LAMPss-E6E7) for cervical intraepithelial neoplasia grade 2/3 (CIN2 and CIN3). Two recombinant baculovirus constructs, Ac-HERV-16-58-18 L1 and Ac-HERV-LAMPss- E6E7, deliver the HERV env and HPV16/58/18 L1 genes and HPV16 E6/E7 to allow transduction of the HPV16/58/18 L1 gene and the HPV16 E6/E7, respectively. In this study, we evaluated the sub-acute toxic effects of the vaccines targeting HPV 16/58/18 and HPV 16. Female ICR mice were treated with a 1:1 mixture of two vaccines (Ac-HERV-16-58-18 L1 and Ac- HERV-LAMPss-E6E7) by repetitive muscular injection once a week for 5 weeks.
Female mice exposed to low, middle, and high doses (1×108, 5×108, and 2.5×109 copies/animal) cervical cancer vaccine did not show mortality, morbidity, or clinical signs (data not shown).
In serum biochemical parameters, only the A/G ratio showed a significant change in all treated groups compared to the control group (Table 3). In some biochemical parameters related to liver (ALT, AST, ALP, and T-Bili) and kidney (BUN, Crea, and T-Bili) function, vaccines did not induce a significant change in the vaccine-treated groups. In addition, in urinalysis (data not shown), necropsy (data not shown), and histopathological effects (Table 4) in the kidney and liver, the vaccines had no distinct toxic symptoms. Therefore, we suggest that the tested vaccines may not induce any toxic responses in the kidney and liver function. The two major proteins in blood, albumin and globulin, are produced in the liver. In this study, the tested vaccines did not show any changes in albumin level (Table 3). However, the A/G ratio was significantly decreased in a dose-dependent manner. We suggest that the decrease in the A/G ratio was due to the response to elevated globulins. Although the A/G ratio is not a specific marker for disease, it has been used as an index of disease state. In particular, a decrease in the A/G ratio due to increased globulin can be induced by some inflammatory diseases, multiple myeloma, collagen disease, and rheumatoid arthritis (http://labtestsonline.org/understanding/analytes/tp/tab/test/). However, although the A/G ratio was significantly decreased in a dose-dependent manner, all values were within normal ranges (0.52-0.62 in ICR mice of 11-12 weeks). In addition, the related pathological symptoms were not present in all treated groups. Therefore, we suggest that the decrease in the A/G ratio in this study may not be considered non-adverse.
Muscular injection of vaccines has been reported to induce mild to moderate injection site symptoms, such as redness, bruising, itching, swelling, pain, and cellulitis [9]. In this study, several female mice exposed to vaccines showed mononuclear cell infiltration at the injection site in all treated groups. In addition, two of 10 mice exposed to high-dose vaccines showed myofiber mineralization. However, because these symptoms could be disappeared and recovered according to stop of injection, we suggest that it could not be considered to be toxic effect to be maintained continuously by vaccine injection.
Some examined animals in the control group showed histopathological effects, such as cystic tubules in the kidney cortex, ectopic thymus in the parathyroid gland, cell infiltration, lymphocytic, focal in salivary gland, dilation, follicular, ectopic thymus in thyroid. However, only one animal exposed to vaccines showed focal ectopic thymus in the thyroid gland, and other histopathological effects were not present in female mice of G1 and G4. In addition, extramedullary hematopoiesis in the spleen was found in both control and high-dose groups (Table 4). Extramedullary hematopoiesis is the normal response during fetal development and can induce an increase in spleen size [17]. In the control and all treated groups, the absolute and relative weight of the spleen did not show any changes (Table 1). Chunduri et al. [18] also reported that extramedullary hematopoiesis can be caused by pathologic processes, such as pulmonary myelofibrosis. However, in this study, all female mice in the control and vaccine-treated groups did not show the related pathologic symptoms. Therefore, the histopathological effects may not be a toxic response caused by vaccine injection.
In conclusion, vaccines targeting high-risk HPV types 16, 58, and 18 did not show any toxicological symptoms for body weight, absolute/relative organ weight, hematological/biochemical parameters, or histopathological parameters in any treated group. In addition, 2.5×109 copies/animal, high exposure dose tested in this study was more than 8,300 fold of expected clinical injection dose, 1×1010 copies/human. Therefore, the tested vaccines may not induce toxic signs at the expected clinical injection dose. Finally, we suggest that the NOAEL of the vaccine following repetitive intramuscular injection for 5 weeks is >2.5×109 copies/animal.
Conflict of interestThe authors have no conflicts of interest with material presented in this paper.
References1. Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003;88(1):63-73.
2. Lee GH, Kang HJ, Kim SY, Park CM. The prevalence of human papilloma virus infections according to Pap smear results in Jeju island. Korean J Obstet Gynecol 2011;54(11):689-695 (Korean).
3. Division of STD Prevention. Prevention of genital HPV infection and sequelae: report of an external consultants’ meeting. [cited 2014 Oct 14]. Available from: http://www.cdc.gov/std/hpv/hpvsupplement99.pdf.
4. Baudu A, Prétet JL, Riethmuller D, Chotard M, Mougin C, Mercier M. Prevalence and risk factors of human papillomavirus infection types 16/18/45 in a cohort of French females aged 15-23 years. J Epidemiol Glob Health 2014;4(1):35-43.
5. Chen Q, Xie LX, Qing ZR, Li LJ, Luo ZY, Lin M, et al. Epidemiologic characterization of human papillomavirus infection in rural Chaozhou, eastern Guangdong Province of China. PLoS One 2012;7(2):e32149.
6. Mishra GA, Pimple SA, Shastri SS. An overview of prevention and early detection of cervical cancers. Indian J Med Paediatr Oncol 2011;32(3):125-132.
7. Herrero R. Human papillomavirus (HPV) vaccines: limited crossprotection against additional HPV types. J Infect Dis 2009;199(7):919-922.
8. Kim KS, Park SA, Ko KN, Yi S, Cho YJ. Current status of human papillomavirus vaccines. Clin Exp Vaccine Res 2014;3(2):168-175.
9. Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012;30 Suppl 5: F123-F138.
10. Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011;365(17):1576-1585.
11. FuturE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356(19):1915-1927.
12. Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a doubleblind, randomised study in young women. Lancet 2009;374(9686):301-314.
13. Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356(19):1928-1943.
14. Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011;364(5):401-411.
15. Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009;302(7):750-757.
16. Block SL, Brown DR, Chatterjee A, Gold MA, Sings HL, Meibohm A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010;29(2):95-101.
Figure 1.Body weight changes (A) and food consumption (B) in female ICR mice treated with cervical cancer vaccines. Each group consisted of 15 animals, and the results are expressed as mean±standard deviation for each group. 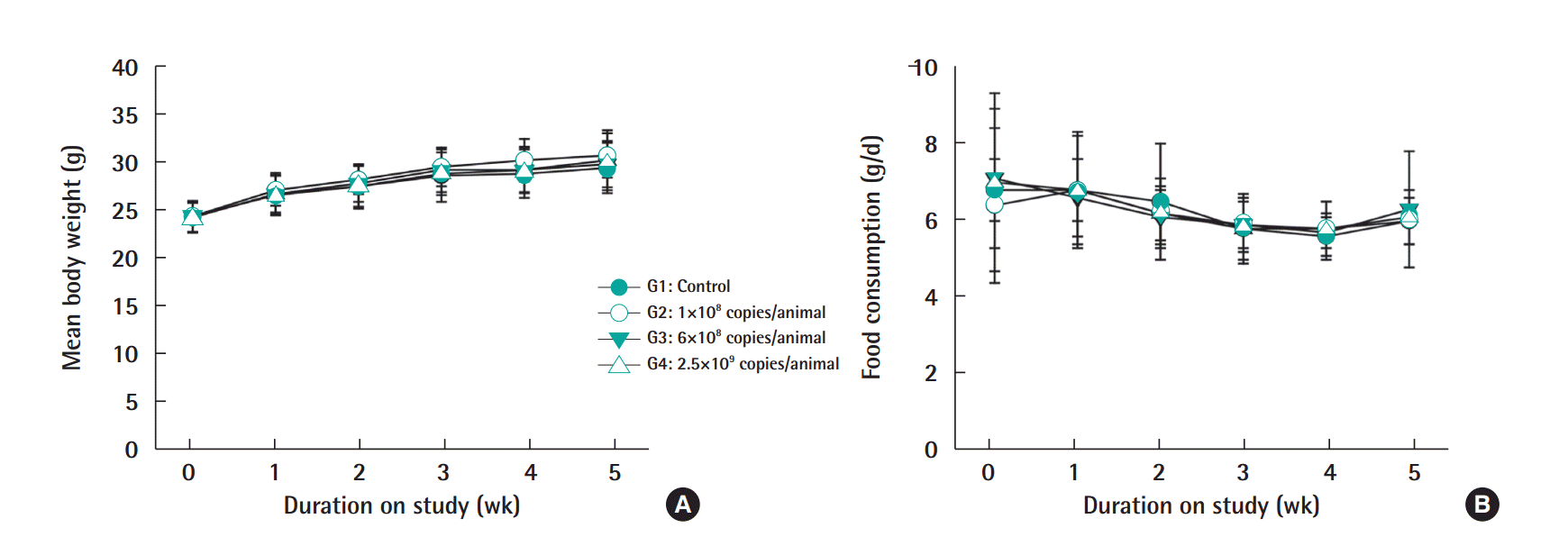
Table 1.Organ weight in female ICR mice treated with cervical cancer vaccines Table 2.Hematological parameters in female ICR mice treated with cervical cancer vaccines RBC, total red blood cell count; Hgb, hemoglobin concentration; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; WBC, total white blood cell count; NUT, neutrophils; LYMPH, lymphocytes; MONO, monocytes; EOS, eosinophils; BASO, basophils; PT, prothrombin time; APTT, activated partial thromboplastin time; G1, control group; G2, 1×108 copies/animal; G3, 5×108 copies/animal; G4, 2.5×109 copies/animal. Table 3.Biochemical parameters in female ICR mice treated with cervical cancer vaccines
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GLU, glucose; BUN, blood urea nitrogen; Crea, creatinine; T-Bili, total bilirubin; T-Chol, total cholesterol; TG, triglycerides; TP, total protein; Alb, albumin; A/G ratio, albumin-globulin ratio; G1, control group; G2, 1×108 copies/animal; G3, 5×108 copies/animal; G4, 2.5×109 copies/animal. Table 4. Histopathological effects at the injection site and in major organs
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||