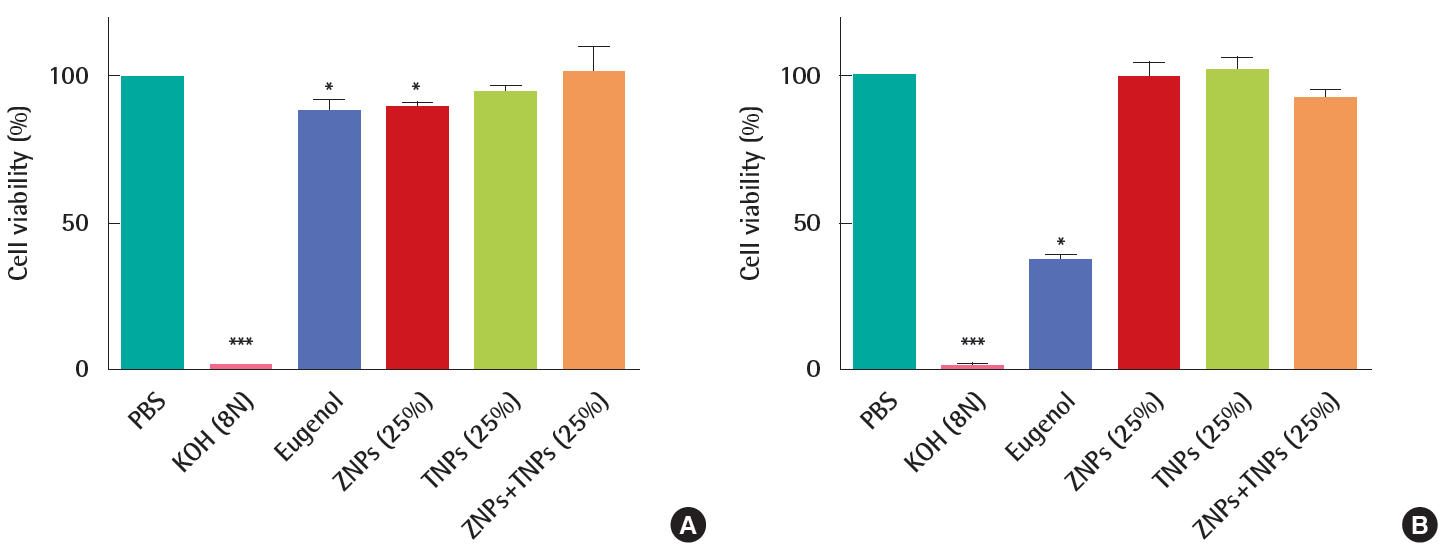Introduction
Zinc oxide nanoparticles (ZNPs) have been used in a variety of products including semiconductors, catalysts and paints. Recently, they are increasingly found in sunscreen products because of the strong ultraviolet (UV) and visible absorption properties of zinc oxide [1-3]. Titanium dioxide nanoparticles (TNPs) have also been widely used as white pigment in paint, UV blocker in cosmetics, welding rod-coating material, disinfectant in environment and wastewater and photosensitizer for the photodynamic therapy [4]. Several studies suggested hazardous possibilities regarding the toxicity of nanoparticles. ZNPs induce genotoxicity in primary human epidermal keratinocytes [5], cause cytotoxicity and enhance inflammatory cytokines levels in murine macrophages [6] and generate reactive oxygen species to kill human bronchial epithelial cells [7]. The repeated application of ZNPs to rat skin with three different doses (75, 180, and 360 mg/kg) for 28 days decreased the collagen content in the skin [8]. ZNPs induced oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to 300 mg/kg for 14 days [9]. An inverse dose-dependent increase was noted in aspartate aminotransferase and alanine aminotransferase when rats were treated with a single oral administration of ZNPs with doses of 5, 50, 300, 1,000, and 2,000 mg/kg [10]. Also many toxicity tests of TNPs have been reported including cytotoxicity in rat neuroglia cells [11], oxidative stress in human keratinocytes [12], acute toxicity in mice [13], histopathology and apoptotic changes in the livers of rats [14] and the effects on reproductive and endocrine system after short-term exposure by oral administration [15]. Although ZNPs and TNPs are widely used in the sunscreen and exposed to human skin, there are still not enough information about skin corrosion and irritation. Furthermore, the mixtures of the two types of nanoparticles as the major formulation of sunscreens have never been tested regarding skin corrosion and irritation.
For a long time, tests of skin corrosion and irritation have been performed based on the test guideline 404 published by the Organization for Economic Co-operation and Development (OECD) [16] in which rabbits are used as test species. The test methods of skin corrosion and irritation using animals are being replaced by alternative methods using in vitro human skin models because of the policy on the animal welfare as well as ethical problems. Now, various 3D models of the human skin are commercially available for the alternative test methods of skin corrosion and irritation. Of them, EpiSkinTM, EpiDermTM and SkinEthnicTM are the most widely used. They make use of reconstructed human epidermis which is obtained from human derived nontransformed epidermal keratinocytes closely mimic the histological, morphological, biochemical and physiological properties of the upper parts of the human skin, i.e., the epidermis [17,18].
Regulatory classifications of chemicals with skin corrosion and/or irritation have been based on rabbit data. However, they are transitioning to in vitro data from alternative methods using human skin models [19]. For the regulatory use of in vitro data using skin models, the skin models should be validated before test [20,21].
In this study, the effects of nanoparticles and their mixture on the skin corrosion and irritation were investigated using the in vitro 3D human skin model KeraSkinTM and the results were compared to those of traditional in vivo data using rabbits.
Methods and Materials
Materials
ZNPs were purchased from Sigma-Aldrich (St. Louis, MO, USA). The nanoparticles were dispersed in deionized water and coated with 3-aminopropyl triethoxysilane to confer a positive surface charge. The pH of ZNPs suspensions was consistently 7.0±0.1 in deionized water and the density was 1.7±0.1 g/mL at room temperature (25°C). According to the information provided by the manufacturer, the average particle size determined by transmission electronic microscopy is less than 35 nm. TNPs (P25, 21 nm-size, Aeroxide®) were kindly provided by Evonik Industry (Ham, France) as part of an OECD program for nanomaterial assessments. According to the information provided by the supplier, titanium dioxide P25 is a fine white powder with hydrophilic character caused by hydroxyl groups on the surface (ca. 5 OH-groups/nm²). It consists of aggregated primary particles. The aggregates are several hundred nm in size and the primary particles have a mean diameter of approximate 20 nm. In this study, TNPs were suspended in deionized water and were sonicated prior to use to ensure a uniform suspension. Size-distribution of ZNPs, TNPs and their mixtures suspended in deionized water were determined using particle sizing system (NICOMP, Santa Barbara, CA, USA).
The 3D human skin model of KeraSkinTM was purchased from Modern Cell & Tissue Technology (Seoul, Korea). This model (diameter 8 mm) consists of normal human-derived keratinocytes, multiple viable cell layers, functional stratum corneum and was validated as a skin model [22].
Cell Culture and Exposure
Based on the recommendation of the supplier, the KeraSkinTM models were transferred onto 6 well plates with 0.9 mL Dullbecco’s modified essential media (DMEM) and pre-incubated in a 5% CO2 incubator overnight. In vitro skin corrosion test was performed based on OECD TG 431 [17]. KeraSkinTM models were exposed to ZNPs, TNPs and their mixture for 3 minutes respectively 60 minutes. The concentrations of nanomaterials were respectively 25% in deionized water and total 30 μL suspension volume was added to the skin model. In the mixture, one volume of 50% ZNPs and one volume of 50% TNPs were mixed to finally produce 25% of nanomaterials and total 30 μL suspension volume was applied to the skin model. Three skin replicates were used for each of the test chemical and control groups. 8N KOH was used as positive chemical and eugenol was used as negative chemical. Also a normal control group was set without test chemical. After exposure, test materials were removed by repeated rinsing with phosphate buffered saline (PBS) and skin models were transferred to the new plates for the viability test.
For the in vitro irritation test, skin models were exposed to nanomaterials for 45 minutes. Sodium dodecyl sulfate (5% solution) was used for the positive chemical. Following exposure, test materials were removed after repeated rinsing with PBS, and post-incubation with new fresh media were performed for 42 hours based on OECD TG 439 [18].
Cell Viability Test
After exposure on a 6-well plate, the skin model was transferred onto a 24-well plate for the cell viability test and 300 μL of 3-(4, 5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) solution (0.3 mg/mL) was added to each skin model. The skins were incubated in a 5% CO2 incubator for 3 hours and insoluble formazan products of MTT were extracted by 2 mL isopropanol per well. A part of the isopropanol extract (200 μL) was transferred onto a 96-well plate to measure the optical density (OD) at 570 nm.
Cytokine Assay and Histopathology
Released IL-1α level was also measured regarding skin irritation in a culture medium of skin model 42 hours post-incubation. Immunoassay kit for human IL-1α was purchased from R&D Systems (Minneapolis, MN, USA). After coating the 96-well plate with capture antibody, media 100 μL obtained from treated skin models were added to the wells and incubated for 2 hours at room temperature. After washing the wells, detection antibody was added to the wells and the plates were incubated at room temperature for 2 hours. Following the washing steps, secondary antibody with streptavidin-horse radish peroxidase was added to the well and the plate was incubated for 20 minutes. After incubation and washing the plate, substrate solution was added and incubated for 20 minutes. After incubation, stop solution containing 2N sulfuric acid was added to the wells and OD was measured at 450 nm using a microplate reader. The concentration was calculated using a standard curve.
For the histopathological examination, 3D human skin or rabbit skin were taken and prefixed in 10% (v/v) neutral buffered formaldehyde. The fixed tissues were trimmed, dehydrated, embedded in paraffin, sectioned, mounted on glass slides, stained with hematoxylin and eosin and examined by light microscopy.
Interpretation of In Vitro Test Results
According to the OECD TG 431 [17], the test material is considered to be corrosive to skin if the viability is less than 50% after 3 minutes exposure. Although the viability after 3 minutes exposure is more than 50%, it is corrosive if the viability is less than 15% after 60 minutes exposure. It will be non-corrosive if the viability is more than 50% after 3 minutes exposure and more than 15% after 60 minutes exposure. Regarding the irritation test, the test material is considered to be irritant to skin if the tissue viability after exposure/post-incubation is less than or equal to 50%. It would be non-irritant if the viability is more than 50% in accordance with OECD TG 439 [18].
In Vivo Dermal Corrosion and Irritation Study
In vivo dermal corrosion and irritation test was performed based on the OECD TG 404 [16]. Rabbits with healthy and intact skin were used (17 weeks of age, about 3.0 kg of body weight). 24 hours before testing, three areas of approximate 6 cm2 were made free of fur using an electrical shaver. Nanomaterials 0.5 g were evenly spread on a lint cloth, applied to the skin and covered with a gauze patch, that was held in place with a non-irritating elastic bandage. If no dermal reaction was observed for the first 3 minutes after patch application, the second patch was applied at a different site and was removed after one hour. If the observation at this stage indicated that an exposure could humanely be allowed to an extend of four hours, a third patch was applied and was removed after four hours. The response was graded according to the guideline.
Results
Size Distribution of Zinc Oxide Nanoparticles, Titanium Oxide Nanoparticles and Their Mixtures
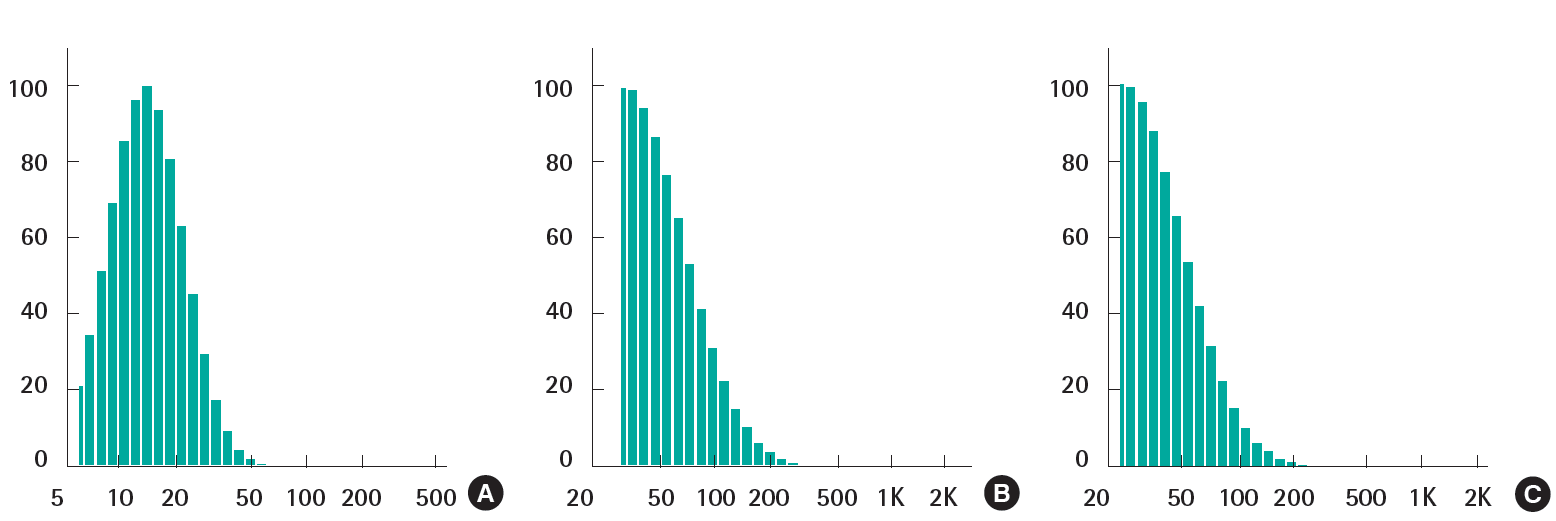
Sized distributions of ZNPs, TNPs and their mixture were measured in deionized water. As shown in Figure 1, the average sizes were 14.9±7.1 nm (ZNPs), 39.4±28.6 nm (TNPs) and 31.1±0.7 nm (mixtures) in 100 ppm suspension. The distribution was not significantly changed when the size distribution was measured in higher or less than 100 ppm (Figure 1). When the test nanomaterials were suspended in other types of vehicles including PBS and DMEM, the stability was changed to aggregate and precipitated because of different ionic strengths of the vehicles.
In Vitro Alternative Skin Corrosion Test
In the corrosion test, the viability of KeraSkinTM which was exposed to positive chemical of 8N KOH for 3 minutes was decreased to 1%. However, the viability was 90±2% in the ZNPstreated group, 94±3% in the TNPs-treated group and 101±9% in the group treated with mixtures of ZNPs and TNPs. No significance was found in TNPs-treated group and mixture-treated group in the statistical analysis. However, significance was found in the decrease of viability in the ZNPs-treated group compared to the PBS treated control group. But the decreased viability in the ZNPs-treated group does not mean corrosion because the viability was still higher than the pre-determined criteria. The viability of negative control group exposed to eugenol which is known to be non-corrosive was 88±4%. The result showed less decreased viability in ZNPs-treated group than in the non-corrosive negative control group (Figure 2A). Although the exposure time was extended to 60 minutes according to the protocol, the viability in the nanomaterial treated groups did not show a significant decrease compared to the control group. The corrosive positive chemical of 8N KOH damaged the cells as seriously as expected and almost all cells were killed. Compared to the exposure time of 3 minutes, the viability decreased to 37±2% in the non-corrosive negative group treated with eugenol (Figure 2B). However it was still higher than the corrosive criteria of 15%.
Based on the interpretation of the results described by OECD TG 431 [17], all the test materials ZNPs, TNPs and their mixture used in this study were found to be non-corrosive because the viability was more than 50% after 3 minutes exposure and more than 15% after 60 minutes exposure.
In Vitro Alternative Skin Irritation Test
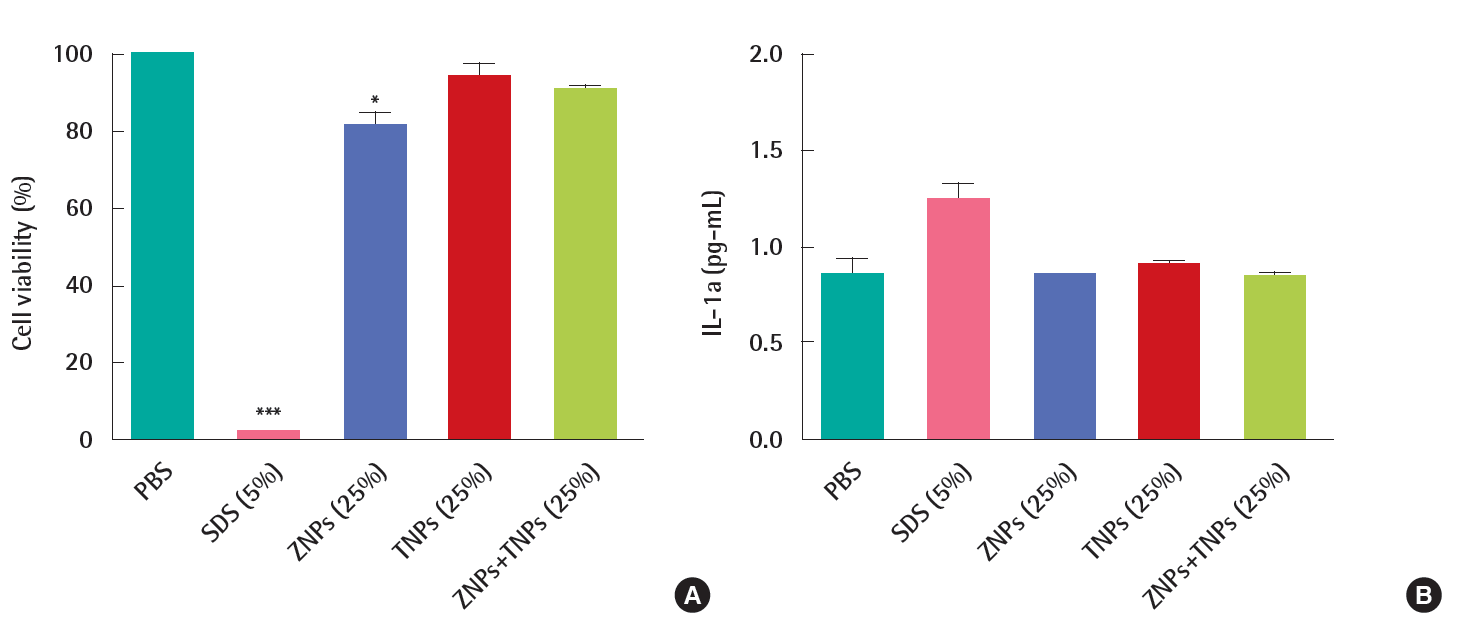
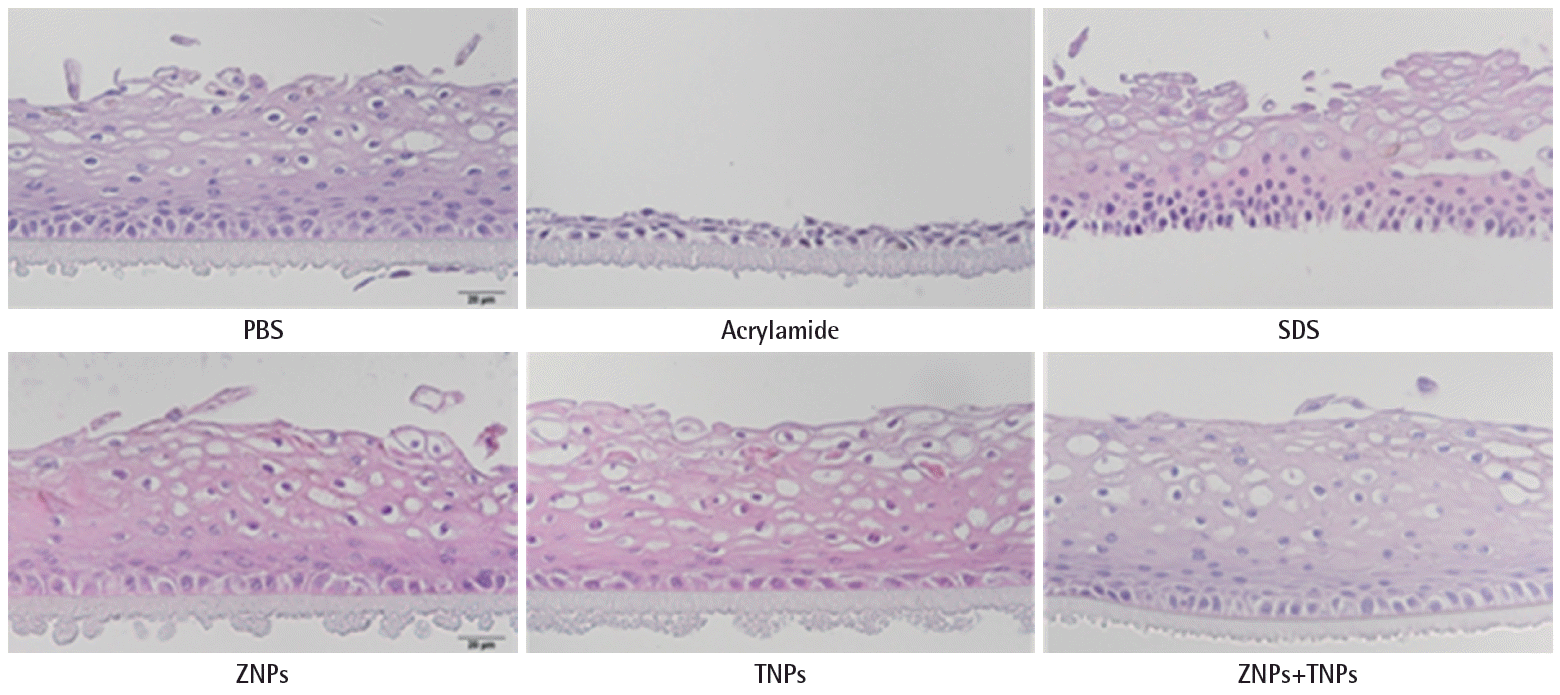
In the irritation test, the cells in the KeraSkinTM models were exposed to positive chemicals of 5% sodium dodecyl sulfate (SDS) for 45 minutes and post-incubated for 42±2 hours. The viability was decreased to 2±0% by SDS. However, the viability was not decreased in the test groups. It was 93±6% in the ZNPs-treated group, 91±1% in the TNPs-treated group and 93±3% in the group treated with a mixture of ZNPs and TNPs. No significance was found in the TNPs-treated group and mixture- treated group in the statistical analysis. However, a significant decrease of viability was found in the ZNPs-treated group when compared to the PBS treated control group (Figure 3A). Although the viability showed a significant difference in the ZNPs-treated group, it did not mean ‘irritant’ because the viability was still higher than the pre-determined criteria 50%. IL-1α which as a marker of inflammation as well as of irritation was also increased in the culture media obtained from the positive control group, 5% SDS (Figure 3B). Compared to the concentration of the PBS treated control group (0.85±0.09 pg/mL), the concentration of IL-1α was increased to 1.25±0.08 pg/mL in the SDS treated group. However, no significant increase was observed in nanomaterial-treated groups, supporting ‘non-irritant’. Histopathologically significant changes were not observed in the skin models treated with test materials (ZNPs, TNPs and their mixture) compared to the control group (PBS treated group), while complete damage and destruction of skin structure was observed in the group treated with corrosive acrylamide. In the skin model treated with irritant positive 5% SDS, the stratum corneum was partly removed and many dead cells were observed attached to the model structure (Figure 4).
Based on the interpretation of the results described by OECD TG 439, all the test materials such as ZNPs, TNPs and their mixtures used in this study were found to be non-irritant because the viability was more than the criteria of 50%. The results were confirmed by IL-1α and histopathological analysis.
In Vivo Study of Skin Corrosion and Irritation
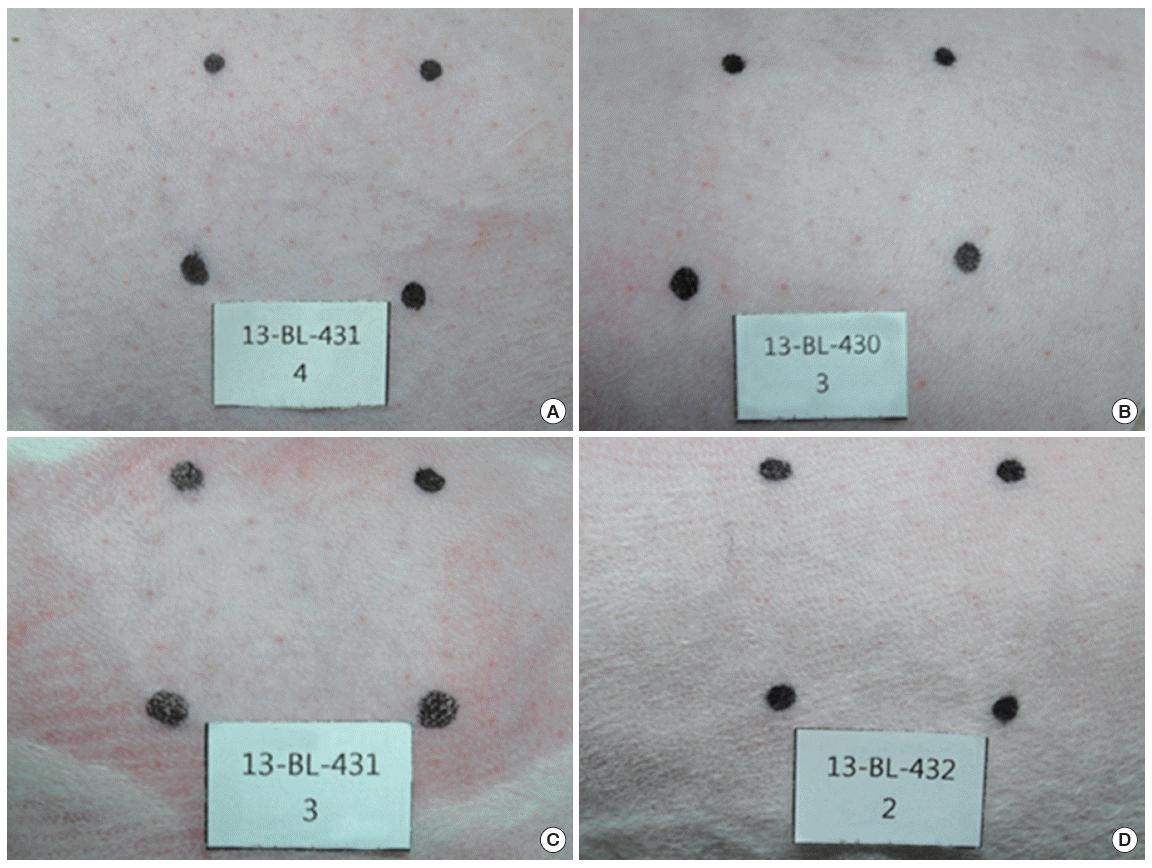
Rabbits were used in vivo for the corrosion and irritation study. When nanomaterials were applied to the rabbit skin, no clinical signs or changes in body weight gain were observed. As shown in Figure 5 no dermal responses including erythema/eschar or edema were found in the skins of rabbits treated with the test materials during the whole experimental period. Also histopathologically significant changes were not observed (Figure 6). Based on the in vivo results, the test materials of ZNPs, TNPs and their mixtures were not corrosive/irritant.
Discussion
Although ZNPs and TNPs are widely used in the cosmetics of sunscreen, the information on the skin toxicity including corrosion and irritation is still not enough [23,24]. Furthermore, more information is necessary about the toxicity of the general formulation of sunscreens [3,25].
The generation of reactive oxygen species (ROS) and cytotoxicity was observed when ZNPs and TNPs were added to suspensions of Balb/c skin cells and then the cells were incubated for 24 hours at 37°C. The nanoparticles coated with triglyceride as an important compound of the skin had less lactate dehydrogenase (LDH) release and ROS generation with higher cell viability, cell metabolic activity and adenosine triphosphate level, compared with pristine nanoparticles [26]. When human-derived keratinocytes were treated with ZNPs and then exposed to UVA for 24 hours, ZNPs significantly induced cytotoxicity and UVA irradiation dose-dependently aggravated the cell death via induction of LDH release and DNA damage [27]. The evidences of apoptosis in human dermal fibroblasts via p53 and p38 and also genotoxicity in primary human epidermal keratinocytes were reported [5,28]. A dermal application of ZNPs showed a decreased content of skin collagen [8].
There was no evidence of TNPs penetration in the viable skin areas when 10% TNPs was applied to the dorsal skin of hairless Wistar Yagi rats once a day for a maximum period of 56 consecutive days. Although focal parakeratosis and spongiosis were observed in the epidermis, the adverse effects by a sub-chronic exposure to TNPs were not significant [29]. UVA irradiation of TNPs induced significant cell damage of human skin keratinocytes mediated by lipid and protein peroxidation. This phototoxicity was mediated by ROS generated during UVA irradiation [30]. However, another publication described that TNPs do not induce phototoxicity, acute cutaneous irritation or skin sensitization in a human skin model [31]. The penetration of nanomaterials of sunscreen formulations was observed when the mixtures of TNPs and ZNPs were applied to UVB-damaged skin, but no transdermal absorption was detected [32].
Although a few reports on the effects of nanomaterials for the skin toxicity have been published yet, it is not easy to find direct evidences on skin corrosion and irritation for the regulatory purpose of chemicals. In the present study we tried to find direct evidences about the mixture of nanoparticles used in sunscreens. ZNPs supplied in 50% (wt) suspension of deionized water were diluted to 25% and TNPs supplied as powder form were suspended in deionized water to 25% for the skin toxicity test. Also the final concentrations of the nanomaterials mixtures were 25%, with the mix of one volume of 50% ZNPs and one volume of 50% TNPs. The concentration of nanomaterials (25%) applied to the skin model was based on the worst case, which is about 100 fold higher compared to that in the formulation of sunscreen products. The stability of the nanomaterials suspended in deionized water was enough to maintain the nanosize particles. The mean size of ZNPs which was known to be less than 35 nm by the supplier was about 15 nm when measured in our laboratory. The mean size of TNPs was about 40 nm, which was bigger than the size suggested by the supplier (20 nm). The size of the mixture of ZNPs and TNPs was 31 nm, close to the size of TNPs. It seemed that there was no size increase due to the interaction between ZNPs and TNPs (Figure 1). ZNPs (25%), TNPs (25%) and their mixtures (25%) were aggregated and precipitated in PBS and DMEM but not in deionized water. According to the OECD test guidelines 431 and 439, test materials are directly applied to the skin model which was submerged into the culture media. A slight aggregation could have been occurred when the suspension in deionized water was applied to the top of the skin. Zeta potentials of ZNPs, TNPs and their mixtures were maintained as positive value and were not significantly changed.
The KeraSkinTM responded well to the positive chemical 8N KOH. Cells seemed to be completely dead after 3 minutes exposure and 60 minutes exposure to KOH (Figures 2A and 2B). Eugenol, 2-methoxy-4-(2-propenyl) phenol, is a clear to pale yellow oily liquid extracted from certain essential oils, especially from clove, nutmeg, cinnamon, basil and bay leaf. It is slightly soluble in water and soluble in organic solvents. According to the OECD TG 431 [33] which is the previous version before revision, eugenol is suggested as a “non-corrosive” reference chemical. When the exposure time was increased to 60 minutes was the viability was decreased to 37±2%, but not to the corrosion criteria 15%. The criteria were sub-classified according to the human skin models in the revised test guideline for in vitro skin corrosion test OECD TG 431. In case of EpiSkinTM, the criteria for corrosion are as follows; corrosive if the viability is less than 35% after 3 minutes exposure, also corrosive if the viability decreased to less than 35% after 60 minutes exposure even if it was more than 35% after 3 minutes exposure, and also corrosive if the viability decreased to less than 35% after 240 minutes exposure even if it was more than 35% after 60 minutes exposure. In cases of EpiDermTM SCT, SkinEthnicTM RHE and epiCS®, the criteria for corrosion are the same in the OECD TG 431 as the previous version before the revision. The viability of negative control group treated with eugenol for 60 minutes was 35%, which is close to the borderline of corrosion using EpiSkinTM. The KeraSkinTM used in this study seemed to be more sensitive than other human skin models because the viability of KeraSkinTM treated with non-corrosive eugenol reached to the borderline of criteria for corrosion. The viability of test group treated with ZNPs for 3 minutes (90±2%) was lower than that for 60 minutes (98±6%) and was not exposure time-dependent. Although statistically difference was shown between the two groups, the viability was much higher than that designated in the criteria. TNPs did not induce cytotoxicity at both, 3 minutes exposure and 60 minutes exposure. The mixture showed the same results (Figures 2A and B). In case of irritation study, the viability of test group treated with ZNPs for 45 minutes was slightly decreased to 93±6%, which is higher than the criteria for irritation, 50% (Figure 3A). The IL-1α level in the media of ZNPs-treated group was not induced compared to the positive control group treated with SDS (Figure 3B). TNPs and the mixture did neither induce cell death nor IL-1α release from the cells, which means no irritation (Figures 3A and B). In this study, UVA irradiation to ZNPs or TNPs was not performed, which may activate the nanoparticles to induce toxicity to model skin.
These results of ‘non-corrosive’ and ‘non-irritant’ were supported by the histopathology of KeraSkinTM model (Figure 4). Acrylamide is known to be a corrosive chemical and it completely damaged the structure of the skin model, while no damages were observed by the exposure to nanomaterials. The SDS-treated group seemed to show mild damages compared to the acrylamide-treated group, but the most cells in outer layers seemed to be dead without nucleus.
Alternative in vitro tests of corrosion and irritation were confirmed by an in vivo test using rabbits. Positive chemicals of corrosion and irritation were not used for human reasons.
Recently, the OECD test guideline of TG 431 was revised and the criteria for corrosion were partly changed based on the human skin models. The corrosion criteria based on the viability of cell were the same, regardless of the skin models. In the revised test guideline, however, commercially available skin models were selected by the validation quality and each skin model has respective criteria for corrosion. Many chemicals were tested for corrosion based on the previous TG 431 (2004) before the adoption of the new revised TG431 (2013) [34]. Also international validation studies were performed with in vitro tests for acute skin irritation and many chemicals were tested [35]. However, no reports have been published yet on skin corrosion and irritation based on the revised guidelines. According to the revised test guidelines TG 431 and TG 439, it seemed very difficult to adopt new skin models because no criteria were described for them including KeraSkinTM.
In summary, TNPs, ZNPs and their mixture were tested for skin corrosion and irritation based on the alternative in vitro method, OECD TG431 and TG439. The results showed that the nanomaterials tested in this study are non-corrosive and non-irritant. The results from alternative in vitro tests were supported by in vivo results using rabbits.