Introduction
Almost 100,000 types of chemicals are currently being distributed around the world. About 2,000 types of new materials are being developed and commercialized per year. Consequently, the volume of chemicals consumed has been continuously increasing. Chemicals are widely used in daily life, such as in household detergents, cars, and electronic devices; however, the safety of only some of these chemicals has been verified. The international management of chemicals has been consistently strengthened through programs such as the European Union's Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) to prevent and reduce the potential impacts on both human health and the environment as the distribution and use of chemicals continue to increase [1]. Korea has cooperated with other member countries of Organization for Economic Cooperation and Development (OECD) since 1999 for the Screening Information Data Set (SIDS) program which has been undertaken by the OECD for chemicals management. The SIDS is the OECD program for conducting initial risk assessments of high production volume (HPV) chemicals manufactured or imported at levels greater than 1,000 tonnes per year based on basic information including physicochemical properties, general information on exposure, human health hazards and hazards to the environment. In addition, it aims to determine whether further evaluation of the potential risk of the investigated chemicals will be needed. From 1993 to January 2012, OECD member countries have evaluated 1,482 chemical substances, and Korea has evaluated 25 chemicals so far. In October 2011, the OECD Cooperative Chemical Assessment Program (CoCAP) was established based on the former SIDS program. The new program undertakes initial risk assessments of existing chemicals that are being used without hazard assessments and presents conclusions and recommendations on their potential risks. Inorganic phosphate, for which the initial risk assessment was performed in 2009 with tricalcium phosphate and in 2011 with calcium hydrogenorthophosphate, plays an important role in biological systems in ecological terms. Phosphate is an important factor which affects the growth rate of organisms by acting as the limiting nutrient to the growth of plants in freshwater environments [2]. However, phosphate causes eutrophication by promoting algal growth in aquatic environments. Eutrophication may occur with excessive phosphates entering into the aquatic ecosystem in the presence of sunlight and nitrogen. Consequently, dissolved in the water is reduced, thus causing organisms to die or decay. Such toxic effects are also related to the release of decay products or direct excretion of toxic substances from sources such as blue-green algae. In addition, phosphates in aquatic environments may help grow introduced plants, causing damage to native plants and thereby changing plant distribution [3]. The major sources of eutrophication vary as follows: fertilizers for agricultural fields, golf courses, and suburban lawns; deposition of nitrogen from the atmosphere; corrosion of nutrients in soil; and water discharged from sewage treatment plants [4]. However, there is insufficient information available on any acute or chronic ecotoxicity and aquatic toxicity assessment of phosphate. Therefore, this study was conducted to evaluate the toxic potential of tricalcium phosphate and calcium hydrogenorthophosphate based on their physicochemical properties and the results of an ecotoxicity test following OECD guidelines for testing of chemicals.
Materials and Methods
Test Substances and Physicochemical Properties
Phosphate, an inorganic compound, is classified by the type of phosphoric acid, including orthophosphate, metaphosphate, diphosphate, and triphosphate; however, it generally refers to orthophosphate. In this study, we conducted assessments on tricalcium phosphate (Ca3O8P2, CAS No.: 7758-87-4) and calcium hydrogenorthophosphate (CaHPO4, CAS No.: 7757-93-9), which had also been evaluated in the SIDS and CoCAP projects. The phosphates used in the study are widely used as foodstuff additives, lubricants, pharmaceuticals and intermediate compounds, antitack agents, synthetic resins, detergents, and disinfectants. According to the data published by the Ministry of Environment in 2006, the volumes of tricalcium phosphate manufactured, used, and imported were 21,600, 1,546, and 76 tonnes, respectively, whereas those of calcium hydrogenorthophosphate manufactured, used, imported, and exported were 1,292, 796, 20,292, and 15 tonnes, respectively [5].
To determine the physicochemical properties of the test substances, the melting point, boiling point, density, vapour pressure, partition coefficient, water solubility, and dissociation constant were obtained from databases including the Merck index [6], CRC handbook of chemistry and physics [7], Hawley's condensed chemical dictionary [8], the pesticide manual, and the European Chemical Agency [9], which were sources recommended in the OECD guidance manual [10].
Ecotoxicity Test
Ecotoxicity tests using fish, Daphnia, and algae were conducted by the good laboratory practice (GLP) facility according to the OECD test guideline (TG) in order to secure reliable data.
Fish, Acute Toxicity Test
The studies were conducted as per OECD TG No. 203: "Fish, acute toxicity test" under the static condition. Test fish aged about 3-4 months living in freshwater were used for the toxicity test. Oryzias latipes, as recommended by the OECD TG, was used because it is widely available and easy to breed. In addition, it is the most used species for hazard assessment of aquatic ecotoxicity. The test fish were found to have a mean total length of 2.0±1.0 cm and fasted for 24 hours immediately prior to exposure. Tap water was filtered to be used as culturing and dilution water. The tap water was passed through a membrane filter (1 µm) to remove particulate matter and through an activated carbon filter to remove the residual chlorine or organic substances. The residual chlorine was below 0.01 mg/L, and the hardness was maintained at 10-250 mg/L as CaCO3. The stability test of tricalcium phosphate and calcium hydrogenorthophosphate in the test medium was performed during the test at nominal concentrations of 10 and 100 mg/L. The samples were taken from the test solution at 1, 24, 48, 72, and 96 hours, and filtered using a 0.45 µm syringe filter to remove insoluble substances. After analyzing calcium concentration from the samples, the concentrations of the test substances were calculated based on measured calcium concentrations and then analyzed using inductively coupled plasma (ICP, Jobin-Yvon Ultima C, Horiba, Japan). The range-finding test was conducted at control, 0.1, 1, 10, and 100 mg/L, and no mortality or adverse effects were observed at 100 mg/L. Based on the results, the definitive test was conducted using the concentrations of control and 100 mg/L (nominal) and a limit test was conducted using 7 fish per test concentration without replication. The temperature was maintained at 23.2-23.9℃. The dissolved oxygen was 5.2-8.5 mg/L, and the pH was 7.13-7.97. The photoperiod was 16 hours of light and 8 hours of dark. No feed or aeration was provided during the test, and the fish were observed daily for abnormality and mortality. The criteria of death employed in this study were the absence of respiratory movement and response to external stimulation. Statistical analysis was not performed and the 50% lethal concentration (LC50) values were expressed as greater than 100 mg/L because no mortality was observed.
Daphnia, Acute Immobilization Test
The studies were conducted following OECD TG No. 202: "Daphnia sp., acute immobilization test" under static conditions. Daphnia magna, less than 24 hours old, was selected as a test species and an M4 medium was used, as recommended by the OECD TG. The temperature was maintained at 20.1-21.1℃, the dissolved oxygen was 7.7-8.9 mg/L, and the pH was 7.8-8.1. The hardness was maintained at 224-243 mg/L as CaCO3. The photoperiod was 16 hours of light and 8 hours of dark. No feed or aeration was provided during the test. The result of the fish acute toxicity test was used as a reference for the stability test of the test substances. The concentrations of the test substances were analyzed at control and 100 mg/L of the test solution using ICP at the beginning (0 hour) and the end (48 hours) of the test. The range-finding test was conducted at control, 0.1, 1, 10, and 100 mg/L. No immobility was observed at 100 mg/L and definitive tests were conducted as a limit test at control and 100 mg/L with 30 Daphnia per concentration (three replicates per concentration). Adverse effects and immobility were examined daily. The criterion of immobilization employed in this study was an inability to swim for approximately 15 seconds after a gentle stir. Statistical analysis was not performed because the limit test was conducted at 100 mg/L (nominal) only. Fifty percent effective concentration (EC50) value was expressed as above the highest test concentration.
Alga, Growth Inhibition Test
An algal growth inhibition test was conducted according to OECD TG No. 201: "Fresh alga and cyanobacteria, growth inhibition test". Unicellular algae with a high sensitivity in toxicity assessment for aquatic ecosystems were selected as a test species. Pseudokirchneriella subcapitata (Strain No. ATCC 22662; UTEX 1648), as recommended by the OECD TG, was used because it is easy to culture and there are sufficient toxicity data available. An OECD culture medium was diluted to produce the biomass of 1×104 cells/mL and then sub-cultured continuously at intervals of 3 to 4 days. For pre-cultivation, the test algae were inoculated into the OECD culture medium at a biomass of 0.5-1.0×104 cells/mL 2 to 4 days prior to study initiation. They were then incubated under the following conditions: 23.1-24.2℃, pH 7.50-9.08, 4,440-8,880 Lux (light intensity), and 100 rpm in a shaking incubator. For the stability test of tricalcium phosphate and calcium hydrogenorthophosphate, the results of fish acute toxicity test were used as a reference. The concentration of the test substances was analyzed using ICP at the beginning (0 hour) and the end (72 hours) of the test. Also, insoluble substances were filtered using a 0.45 µm syringe filter.
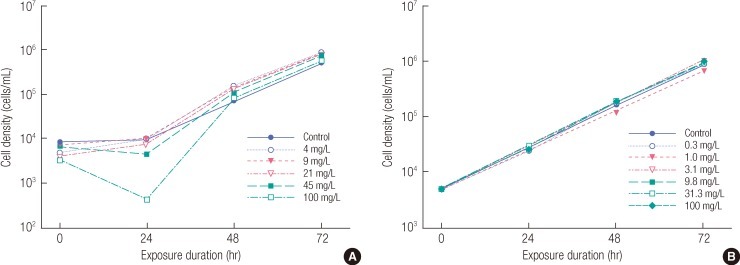
The range-finding test of tricalcium phosphate was conducted at control, 0.1, 1, 10, and 100 mg/L, and the percent inhibition of growth rates was 19% for the average specific growth rate and 59% for the area under growth curve method at the highest dose of 100 mg/L. Therefore, the definitive test was conducted using the nominal concentrations of control, 4, 9, 21, 45, and 100 mg/L, with the highest concentration of 100 mg/L and a factor of 2.2. The range-finding test of calcium hydrogenorthophosphate was conducted at control, 0.1, 1, 10, and 100 mg/L, and the percent inhibition of growth rates was 13.5% for the average specific growth rate and 51.4% for the yield at the highest dose of 100 mg/L. Therefore, the definitive test was performed using the nominal concentrations of control, 0.3, 1, 3.1, 9.8, 31.3, and 100 mg/L, with a factor of 3.2. Morphological changes of algal cells were observed including expansion, aggregation, atrophy, and decoloration at 72 hours after exposure. Statistical analysis was not performed, since less than 50% of growth inhibition at 100 mg/L (nominal) was observed in the test and therefore, the EC50 values were expressed as above the test concentration.
Results
Physicochemical Properties
Tricalcium phosphate is an odorless and tasteless amorphous substance, with the melting point of 1,670℃, the density of 3.14 g/cm3, and low solubility below 20 mg/L (20℃) in water (Table 1). Calcium hydrogenorthophosphate is an odorless and tasteless white crystallized powder with the melting point of >450℃. Its density is 2.92 g/cm3 and water solubility is 153 mg/L (20℃, pH 6.5) in water (Table 1). The boiling point, vapour pressure, partition coefficient, and dissociation constant are not applied to inorganic phosphates such as tricalcium phosphate and calcium hydrogenorthophosphate. Detailed physicochemical properties are shown in Table 1.
Ecological Toxicity
Fish, Acute Toxicity Test
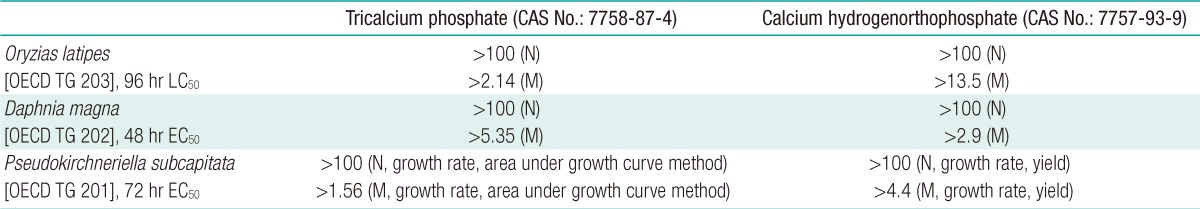
In the stability test of tricalcium phosphate during 96 hours, the mean calcium concentration at control was 13.2 mg/L and measured calcium concentrations at 10 mg/L (nominal) were 13.0-13.9 mg/L, showing that both values were in similar ranges. The calculated test concentrations ranged between 0.27 and 1.87 mg/L, which were 2.7-18.7% of the nominal concentrations. Therefore, tricalcium phosphate is considered as a sparsely soluble substance. The concentrations of tricalcium phosphate were similar to the measured calcium concentrations at control and 100 mg/L (nominal) during the test. The concentrations of the test substance were calculated to be 0.53-2.14 mg/L based on measured calcium concentrations. The results of the fish acute toxicity test showed that no mortality or adverse effects were observed at control or 100 mg/L (nominal). The 96 hr LC50 and no observed effect concentration (NOEC) in O. latipes were >100 mg/L (measured concentration: >2.14 mg/L) (Table 2).
In the stability test of calcium hydrogenorthophosphate, the concentrations of the test substance at 100 mg/L (nominal) were calculated to be 2.3-8.8 mg/L based on measured calcium concentrations during 96 hours. The measured concentration of the test substance generally increased over time according to the measured calcium concentration at control. Based on the results, stability was verified because the concentration of the test substance was not decreased in the test solution during the stability test. The concentrations of the test substance were measured to be 13.0-14.2 mg/L at control and 100 mg/L (nominal) at 0, 48, and 96 hours. The results of the fish acute toxicity test showed that no mortality or adverse effects were observed in any of the test concentrations during the test. The 96 hr LC50 and NOEC of calcium hydrogenorthophosphate were >100 mg/L (measured concentration: >13.5 mg/L) in O. latipes (Table 2).
Daphnia, Acute Immobilization Test
The concentrations of tricalcium phosphate were analyzed at control and 100 mg/L (nominal) at 0 and 48 hours. The results suggested that the test substance was insoluble and its concentration was similar to that of the control. Therefore, it was difficult to determine the concentration of the test substance. The concentrations of calcium ion were measured to be 80.3-87.1 mg/L at control, which is attributed to calcium ion (CaCl2 · 2H2O) present in the M4 culture medium. The concentration of the test substance was calculated using the concentration of calcium excepting the mean calcium concentration at control. The results suggested that the concentration of the test substance was significantly lower than nominal, and the test substance was insoluble in water. Therefore, all the test results were expressed as nominal concentration. No immobility or adverse effects were observed at control or 100 mg/L (nominal). The 48 hr EC50 of tricalcium phosphate was >100 mg/L (measured concentration: >5.35 mg/L) in Daphnia (Table 2).
For calcium hydrogenorthophosphate, its mean concentration was analyzed to be 2.75 mg/L at 100 mg/L (nominal) at 0 and 48 hours. In the acute toxicity test with Daphnia, no immobility, adverse effects or abnormal behavior were observed at control or 100 mg/L (nominal). The 48 hr EC50 was >100 mg/L (measured concentration: >2.9 mg/L) (Table 2).
Alga, Growth Inhibition Test
The concentrations of tricalcium phosphate were analyzed at control, 4, 9, 21, 45, and 100 mg/L at 0 and 72 hours, and the results indicated that the test substance was sparsely soluble in water. The mean concentrations of the test substance were 0.08-0.88 and 0.61-1.56 mg/L at 0 and 72 hours, respectively, ranging between 0.8 and 15.3% of the nominal concentrations. The concentration of the test substance was calculated using the concentration of calcium excepting the mean calcium concentration at control, because calcium ion was present in the culture medium and therefore measured at control. Consequently, the concentration of the test substance was, relatively, significantly low at 0 and 72 hours. Therefore, all the test results were expressed as nominal concentration because the test substance was insoluble in water. Algal cells were observed after 72 hours of exposure, and the results showed that there were no morphological changes at control, maintaining a semicircular shape. The growth curves for cell density measured in triplicate every 24 hours in each concentration of the test substance are shown in Figure 1A. In all the test concentrations, the growth rates of algal cells were higher than those of the control. Therefore, it was determined that the test substance would cause growth stimulation rather than growth inhibition. The cell density measured at 100 mg/L at 24 hours was considered to show an experimental error due to variables from the test using living organisms. In the algal growth inhibition test, the 72 hr EC50 was calculated to be >100 mg/L (measured concentration: >1.56 mg/L) using the average specific growth rate and the area under the growth curve method (Table 2).
Calcium hydrogenorthophosphate was insoluble in the OECD culture medium and therefore the concentration of the test substance was detected to be significantly low during the test. The measured concentrations of the test substance were 0.3, 0.4, 0.3, 0.8, 1.7, and 4.4 mg/L (nominal concentrations: 0.3, 1.0, 3.1, 9.8, 31.3, and 100 mg/L) at the beginning of the test (0 hour). At 72 hours, the test substance in the test solution was not detected at 0.3, 1.0, and 3.1 mg/L (nominal), but its concentrations at 9.8, 31.3, and 100 mg/L (nominal) were estimated to be 0.3, 1.6, and 4.7 mg/L. As in the case of tricalcium phosphate, algal cells were observed to maintain a similar shape to the control in all test solutions, and microorganisms other than algae were not observed. The growth curves for cell density measured in triplicate every 24 hour in each concentration of the test substance are shown in Figure 1B. The 72 hr EC50 was estimated to be >100 mg/L (measured concentration: >4.4 mg/L) using the average specific growth rate and yield (Table 2).
Discussion
The results of the acute toxicity tests of tricalcium phosphate and calcium hydrogenorthophosphate with fish and Daphnia and the algal growth inhibition test are shown in Table 2. Both substances have low solubility in water. The concentrations of tricalcium phosphate and calcium hydrogenorthophosphate measured at 100 mg/L (nominal) were 0.53-2.14 mg/L and 13.0-14.2 mg/L, respectively, in all three test groups during the test. Therefore, when poorly water-soluble substances are used in ecotoxicity tests with fish, Daphnia and algae, additional guidance on toxicity test would be recommended for determining the toxicity more accurately.
In this study, the endpoints were expressed as the nominal concentration in terms of the loading rate and measured concentration (i.e., the highest concentration measured in solution). As a result, the L(E)C50 in the acute toxicity tests of fish and Daphnia and the algal growth inhibition test was > 100 mg/L (nominal). The endpoints for fish, Daphnia, and algae were evaluated according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) [12] and consequently not subject to GHS categories for substances hazardous to the aquatic environment (Table 3). No previous studies have been performed with the same substances. However, in the aquatic toxicity tests of phosphoric acid, potassium salt (2:3), dehydrate with fish (Oncorhynchus mykiss), invertebrates (Daphnia magna) and algae (Desmodesmus subspicatus), the L(E)C50 was >100 mg/L which is analogous to the endpoints in this study [13]. Therefore, the acute toxicity effects of tricalcium phosphate and calcium hydrogenorthophosphate are considered minimal in aquatic ecosystems. Chronic toxicity tests should be conducted for more precise ecotoxicity assessment.
In this study, physicochemical properties were investigated and ecotoxicity tests were conducted to evaluate the toxic potential of phosphate. The results of the ecotoxicity tests of tricalcium phosphate and calcium hydrogenorthophosphate are as follows: In the acute toxicity tests with fish, the 96 hr LC50 was >100 mg/L (measured concentration: >2.14 mg/L) and >100 mg/L (measured concentration: >13.5 mg/L), respectively. In the acute toxicity tests with Daphnia, the 48 hr EC50 was >100 mg/L (measured concentration: >5.35 mg/L, tricalcium phosphate) and >100 mg/L (measured concentration: >2.9 mg/L, calcium hydrogenorthophosphate). In algal growth inhibition tests of tricalcium phosphate and calcium hydrogenorthophosphate, the 72 hr EC50 was >100 mg/L (measured concentration: >1.56 mg/L) and >100 mg/L (measured concentration: >4.4 mg/L), respectively.
Based on these results, it was concluded that phosphate, with a nominal concentration of above 100 mg/L, possessed no toxicity in aquatic organisms. In general, the total phosphorus concentration including phosphate in rivers and lake reaches levels of several ppm, suggesting that phosphate has toxic effects. However, excessive inflow of phosphate into aquatic ecosystems has the potential to cause eutrophication due to algal growth. Therefore, relevant chemicals management measures are needed.