In vitro acute inhalation toxicity for TiO2 (GST) using 3D human tissue model (EpiAirway™)
Article information
Abstract
The present study was performed to screen in vitro potential acute inhalation toxicity using an EpiAirway™ tissue model (human tracheal/bronchial tissue) for the nano-sized titanium dioxide, GST manufactured as a photocatalyst through of sludge recycling and to compare with P-25 a commercialized photocatalytic material. According to the protocol provided by in vitro tissue manufacturer, the GST was exposure to the tissue for 3 hours in 450, 500, 650, 850 mg/mL concentration after preliminary dose range finding study and then tissue viability (%, IC75) was calculated using the MTT assay. Besides, the histopathological observation was performed to compare to the MTT assay. As a result of study, IC75 could not be confirmed at 850 mg/mL in both GST and P-25 and the grade was confirmed to be IC75> 600 mg/mL in vitro model tissue category. Therefore, it was considered that the GHS category could be classified as ‘No classification’ in screening method for potential acute inhalation toxicity. Also, not the morphological effects of epithelial cells in tissue model were observed compared with the vehicle control and histological findings were similar to the results of MTT Viability assay. Based on these results, the potential acute inhalation toxicity for GST produced through sludge recycling using in vitro tissue model inhalation toxicity showed that it could be non-hazardous substance. However, further study (in vivo study, etc.) is thought to be needed to ascertain whether GST is a toxic effect or safe.
Introduction
The TiO2 nanoparticles (NPs) has been widely used in a variety of products including consumer products, paints, pigments, orthodontic composites, food and cosmetic products [1–4]. In addition, TiO2 NPs are produced abundantly and used widely because of high stability, anticorrosive and photocatalytic properties [5–6].
For this reason, the toxicological studies of TiO2 NPs have been currently performed with several routes including dermal, oral and inhalation [7–14]. Especially, it is important to assess the risk of respiratory tract serves as both target tissue and portal of entry to the systemic circulation for inhaled substances. In inhalation toxicity study, most of the studies have been reported with the P25 from, which consists of TiO2 NPs (anatase structure: 80~90%, rutile structure: 20~10%), generally used in catalytic and photocatalytic industrial application [7].
In test guidelines, the study of acute inhalation toxicity potential is important for establishing safe handing, package, labeling, transport, and use procedures for chemicals. The commonly accepted test of acute inhalation for GHS (Globally Harmonized System) category has been in vivo animal study; OECD test guidelines 403 (acute inhalation toxicity test), 433 (acute inhalation toxicity: Fixed concentration procedure), 436 (acute inhalation toxicity: acute toxic class method) [15–17]. However, the limitations in animal study have been demonstrated for interspecies differences in the respiratory tract architecture and function and it is difficult that the conclusions are drawn on the potential hazard of inhaled compounds in human [18]. Recently, a new test for determining acute inhalation toxicity has been brought forward for validation and OECD adoption. This test adopts the EpiAirway™ model, a ready-to-use, three-dimensional (3D) in vitro muco-ciliary tissue model consisting of normal, human-derived tracheal/bronchial epithelial cells cultured at the Air-Liquid Interface (ALI) and pre-validation results for 59 chemicals showed that it was a promising human-relevant in vitro alternative to animal tests for acute inhalation toxicity studies. This pre-validation data indicated that GHS acute inhalation toxicity category 1–2 had IC75 toxicity values < 150 mg/mL, category 3 had IC75 toxicity values 150 mg/mL < IC75≤400 mg/mL, category 4–5 had IC75 toxicity values 400 mg/mL < IC75≤ 600 mg/mL based on combined GHS acute inhalation plus STOT (Specific Target Organ Toxicity) [19]. Chemicals that have been evaluated and determined to be nonhazardous has IC75 toxicity values> 600 mg/mL.
Recently, the study was conducted to produce TiO2 from the wastewater sludge generated by the flocculation of secondary wastewater with titanium tetrachloride (TiCl4) [20]. Also, Gong JH et al. conducted a study that TiO2 photocatalyst was produced from the precipitated sludge using TiCl4 coagulants in the sewage treatment plants (Changwon-si, Gyeongsangnam-do, Republic of Korea) and it had cost-competitive lower than price of commercial photocatalyst (P-25) [21].
The aim of the present study was conducted using in vitro human 3D model (EpiAirway™) as a screening method for the potential acute inhalation toxicity for TiO2 photocatalyst (GST) produced through sludge recycling of the sewage treatment plants. Also, we made a comparison with P-25 form, the most commonly tested commercial substance and the histopathological examinations were performed for model tissue.
Materials and Methods
Test substance
The GST (pale yellow powder) was manufactured from the precipitated sludge using TiCl4 coagulants in the sewage treatment plants and P-25 (commercial nano-particle products, white powder; Evonic, Germany) was provided by Bentech Frontier Co. Ltd. The crystalline composition in X-ray diffraction (XRD) analysis was performed by the Research Institute for Catalysis in Chonnam National University and the crystalline ratio was 100% anatase and 88% anatase / 12% rutile in GST, P-25, respectively.
The measurements of zeta potential, particle size image (SEM, scanning electron microscope) and TEM (Transmission Electron Microscopy) image, size distribution for GST were performed as follows and were presented in (Figure 1 and Figure 2), respectively. In zeta potential, it indicated negative zeta potential value (−35.4 mV), which is an indirect parameter for surface charge. A more negative zeta potential favors the particle agglomeration less rapidly. This value showed that the charge of the hydrated particles was not high enough to repel each other by electrostatic forces. Also, the measured particle sizes including size distribution indicated that the particles of GST had a distribution of various size (95.8±46.3 nm, >100 nm-34.1%).
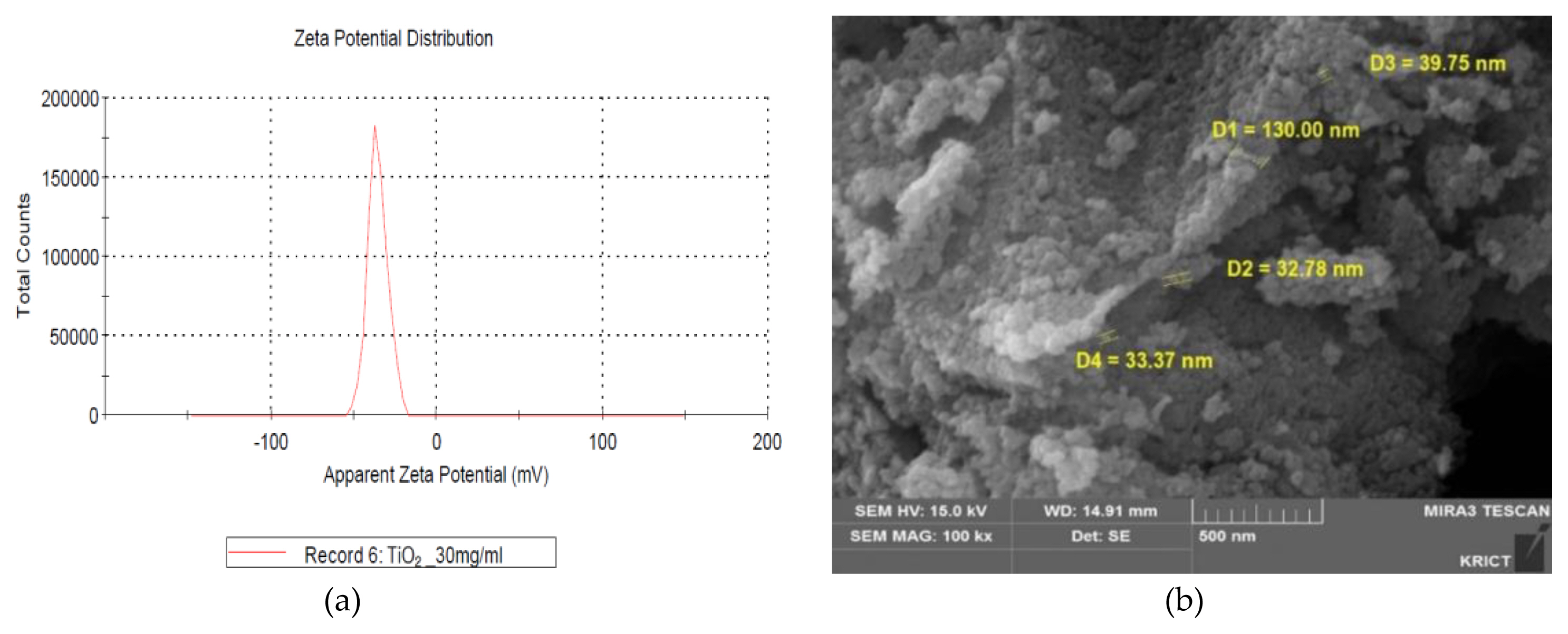
Characterization of TiO2 particles (GST): (a) Zeta potential (−35.4 mV, 30 mg/mL); (b) SEM (scanning electron microscope) image.

Characterization of TiO2 particles (GST): (a) A particles dispersed in 99.9% EtOH was deposited on a copper grid and analyzed by TEM (Transmission electron microscope) image; (b) Size distribution of the imaged GST. The diameter measured was 95.8±46.3 nm (mean±standard deviation) by ImageJ and 34.1% by number of the particles had a diameter>100 nm.
For zeta potential for surface analysis – particle size & zeta potential analyzer (Zetasizer Nano ZSP, Malvern Instruments LTD., UK) in Korea TECH
For particle size analysis - FE-SEM(Field Emission Scanning Electrong Microscope, Tescan Corp., Czech) equipped with EDS systems (Thermo scientific, USA) in KRICT (Ulsan department)
For TEM image – FE-EF-TEM (Field Emission Energy Filtered Transmission Electron Microscopy, JEOL, Japan) in Korea Basic Science Institute (Jeonju department)
For size distribution analyze of TEM image – ImageJ software (https://imagej.nih.gov/ij/download.html)
In addition, the acetonitrile (Burdick and Jackson., Cas No. 75-05-8), one of 59 chemicals used for pre-validation study was selected to verify reproducibility of IC75 before performing the study of TiO2 NPs (GST / P-25, Cas No. 134363-67-7) [19]. In the confirmation test for the vehicle, GST and acetonitrile were identified as sterile distilled water (D.W.), and P-25 was 20% acetone (SIGMA-Aldrich, Lot No.: MKCK2599)/olive-oil (SIGMA-Aldrich, Lot No.: BCBT7822) (1:4 v/v%). The TiO2 NPs were suspended in vehicles and it was used after stirring and additional sonication to disperse. As a positive control, formaldehyde solution (SIGMA-Aldrich, Lot No.: 47033-U, ≥36.0% in H2O) was used for TiO2 NPs (GST, P-25).
The sodium dodecyl sulfate solution (SDS) was selected considering to be a substance known as irritant for inhalation, skin, eye [22] and 10% SDS (SIGMA-Aldrich, Lot No.: BCCB7687) was diluted to 5% (v/v) in PBS (Phosphate Buffered Saline, Lonza Ltd., Lot No. 0000725114) and then used as positive control of acetonitrile [23]. Vehicles were used as the negative control group (D.W., acetone/olive-oil (1:4)).
3D In vitro respiratory tissue model (EpiAirway™)
Preparation of tissue
The EpiAirway™ tissue model was produced by MatTek Corporation (Ashland, MA, USA) and purchased through Dail Scientific Trade, Inc. in Korea. The components included tissue (AIR-100), assay kit (assay medium; AIR-100-ASY, AIR-100-MM, buffer; TEER Buffer), and MTT kit (diluent; MTT-100-DIL, concentrate; MTT-100-CON, extractant; MTT-100-EXT). After receiving the tissue, the insert membrane was separated from agarose and was transferred in to 6 well plate. Then 1.0 mL of cold medium was dispersed into each well of the 6-well plates provided and the tissue was equilibrated overnight (37°C, 5% CO2 16–18 hours).
Preliminary dose range finding (DRF) test
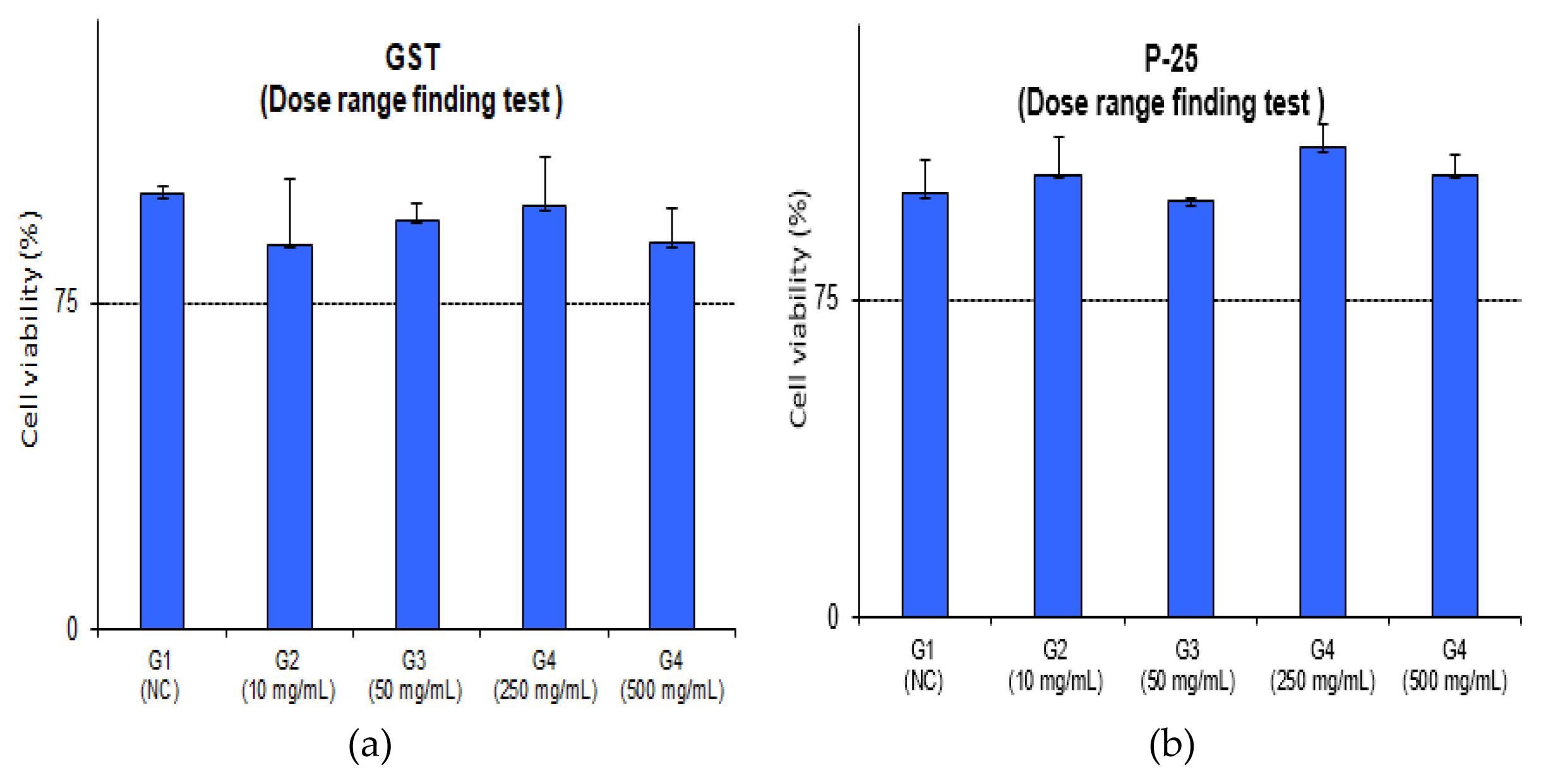
The test was carried out in accordance with the EpiAirway™ toxicity testing protocol. 100 uL of test substance were applied to the apical surface of each tissue; 0 (vehicle only), 10, 50, 250 and 500 mg/mL. The tissues with test substance were incubated for 3 hours (37°C, 5% CO2 incubation, n=3 tissue/dose) and then the MTT viability for IC75 value was performed. In the case of acetonitrile, the DRF test was omitted, referring to the pre-validation data (IC75; 284.72±72.1 mg/mL) [19].
Definitive toxicity test
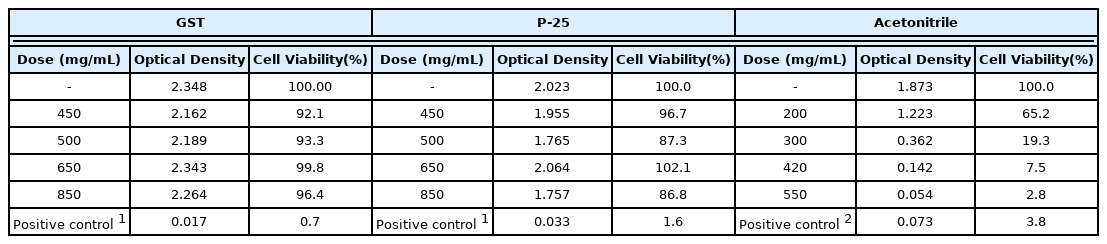
Aa a result of the DRF test, the viability for TiO2 NPs, GST and P-25 was IC75>500 mg/mL and definitive doses were determined in accordance with the EpiAirway™ toxicity testing protocol; 0 (vehicle only), 450, 650, 850 mg/mL (Table 1). In addition, one dose (the high dose of the DRF, 500 mg/mL) was included to check the reproducibility.
The formaldehyde was determined as a positive control according to the EpiAirway™ toxicity testing protocol. In Acetonitrile, the determined doses were 0 (negative control only), 200, 300, 420, and 550 mg/mL considering IC75 data of pre-validation. The detailed dose determination is explained in (Table 1). The test substances were treated in the same way as preliminary dose range finding test; incubation for 3 hours (37°C, 5% CO2 incubation, n=3 tissue/dose) and MTT assay for viability.
MTT viability assay
EpiAirway™ tissues (Air-100) were transferred to 24-well culture plates containing 300 uL of MTT reagent per well and incubated in a 37C, 5% CO2 incubator for 3 hours. After incubation, the tissues were submerged in 2.0 mL of MTT extractant and extracted into shaker for 2 hours. For extraction time the plate was sealed to protect against light and prevent evaporation of MTT extractant. And then 200 uL of extractant from each tissue was transferred to a clear 96-well plate and absorbance was measured at 570 nm with background at 650 nm subtracted.
The viability of test substance-exposed tissues was calculated relative to negative controls using the equation: Relative viability (%) = [OD test substance / Mean OD negative control]×100. All control and treatment groups included n=3 replicates. Also, GraphPad Prism software was used to calculate the inhibitory concentration values (IC75; the dose required to reproduce the EpiAirway™ culture viability to 75% of vehicle-treated tissues) were calculated using Prism software (USA).
Histology
Histopathological examination was performed to observe morphological changes in tissues according to cell viability. For this, one tissue from each treatment group, including the negative control tissues, was fixed in 10% neutral buffered formalin (BBC Biochemical, USA, Lot No.95922) (overnight, room temperature) and the tissues were embedded with paraffin. The tissue sections were adhered to slides and were stained by hematoxylin (BBC Biochemical, USA, Lot No.95925) and eosin (BBC Biochemical, USA, Lot No.959058) (H&E) for observation under light microscopy.
Results and Discussion
Preliminary dose range finding (DRF) test
The average MTT viability of GST was 88.5%, 94.1%, 97.3%, 88.8% and P-25 was 104.6%, 98.4%, 110.9%, 104.6% in concentration of 10, 50, 250, 500 mg/mL, respectively. As a result of viability, TiO2 NPs (GST and P-25) had a MTT viability of 75% higher. Also, the optical density values were 2.0 or higher [negative control optical density should be ≥ 1.455: value – 2.579 (GST), 2.084 (P-25)]. These results indicate that there was no difference between the GST and P-25. IC75 could not be calculated that cell viability was observed more than 75% in all doses. The detailed evaluation of relative viability and optical values for the preliminary dose range finding test is depicted and detailed in (Figure 1, Figure 2 and Table 2).
Definitive toxicity test
The average MTT viability of GST was 92.1%, 93.3%, 99.8%, 96.4% and P-25 was 96.7%, 87.3%, 102.1%, 86.8% in concentration of 450, 500, 650, 850 mg/mL and the positive control (formaldehyde) was 0.7%, 1.6%, respectively. The results of the MTT Viability assay for GST and P-25 meant that IC75 values could not be calculated at 850 mg/mL (high dose) and the grade was IC75 > 600 mg/mL in EpiAirway™ in vitro model considering as follows.
Although EpiAirway™ in vitro model has not yet been adopted as OECD test guideline for acute inhalation toxicity, it was considered that the study using IC75 value could be a screening method for GHS categorization. Therefore, it was expected that the category for GST would not be classified, and no potential toxicity was considered.
In the case of P-25, commercial product, the safety data sheet (SDS) of manufacturer (Evonik Corp.) showed that LC50 (rat) was > 6.82 mg/L in dusts, mists and fumes vapour route. It indicates that GHS classification was considered to be category 3 (2.0 < acute toxicity estimate≤ 10.0, vapour) or category 5 (dusts, mists). Based on the these, GST substance was considered to be similar with P-25 in inhalation route and further study (in vivo study, etc.) is thought to be needed to ascertain whether GST shows a toxic effect or safe.
The inhaled nanoparticles are mainly found in the upper airways (nose, mouth, pharynx, larynx and trachea) but it can reach the deeper lungs and deposit in alveoli [24]. In the study for assessment pulmonary effects, three studies for rodents reported irritation and mild or moderate pulmonary inflammation [12–14]. Also, the study using mice (8.88 mg/m3 for 4h/day) showed higher counts of total cells and alveolar macrophage in the BALF (bronchoalveolar lavage fluid) [25] and the study exposed to aerosols in rats (2, 10, 50 mg/m3 for 6hr/day) showed a lung inflammation with dose-dependent increases in BALF [26]. In this study, we performed this study to screen the potential acute inhalation toxicity for GST using the EpiAirway™ model (tracheal/bronchial tissue) and this result indicates that TiO2 NPs, GST produced through sludge recycling is considered to be safe comparing with the reference studies.
In acetonitrile test, the average MTT viability was 65.2%, 19.3%, 7.5%, 2.8% in concentration of 200, 300, 420, 550 mg/mL and IC75 was calculated as 183.6 mg/mL. This result showed that grade was 150 < IC75 ≤400 mg/mL in 3D in vitro model and GHS was considered to be category 3. Though GHS acute classification was known for category 4 (in vivo, in male CD-1 mice, LC50 2,693 ppm), it was identified in the same category 3 in 3D in vitro model as the pre-validation data (IC75: 284.72±72.1 mg/mL [19, 27]. The difference in a 3D model based on GHS-STOT classification and in vivo data for GHS category was considered to be due to the difference in species; GST-STOT classification based on the human data and tissue with human-derived tracheal/bronchial epithelial cells in EpiAirway™ model, animal data (mouse) in vivo study.
The detailed evaluation of relative viability and optical values for the Definitive toxicity test of relative viability is depicted and detailed in (Figure 3 and Table 3). The positive control (SDS) was 3.8% cell viability and it showed the strong irritation for tissue model.
Histopathology
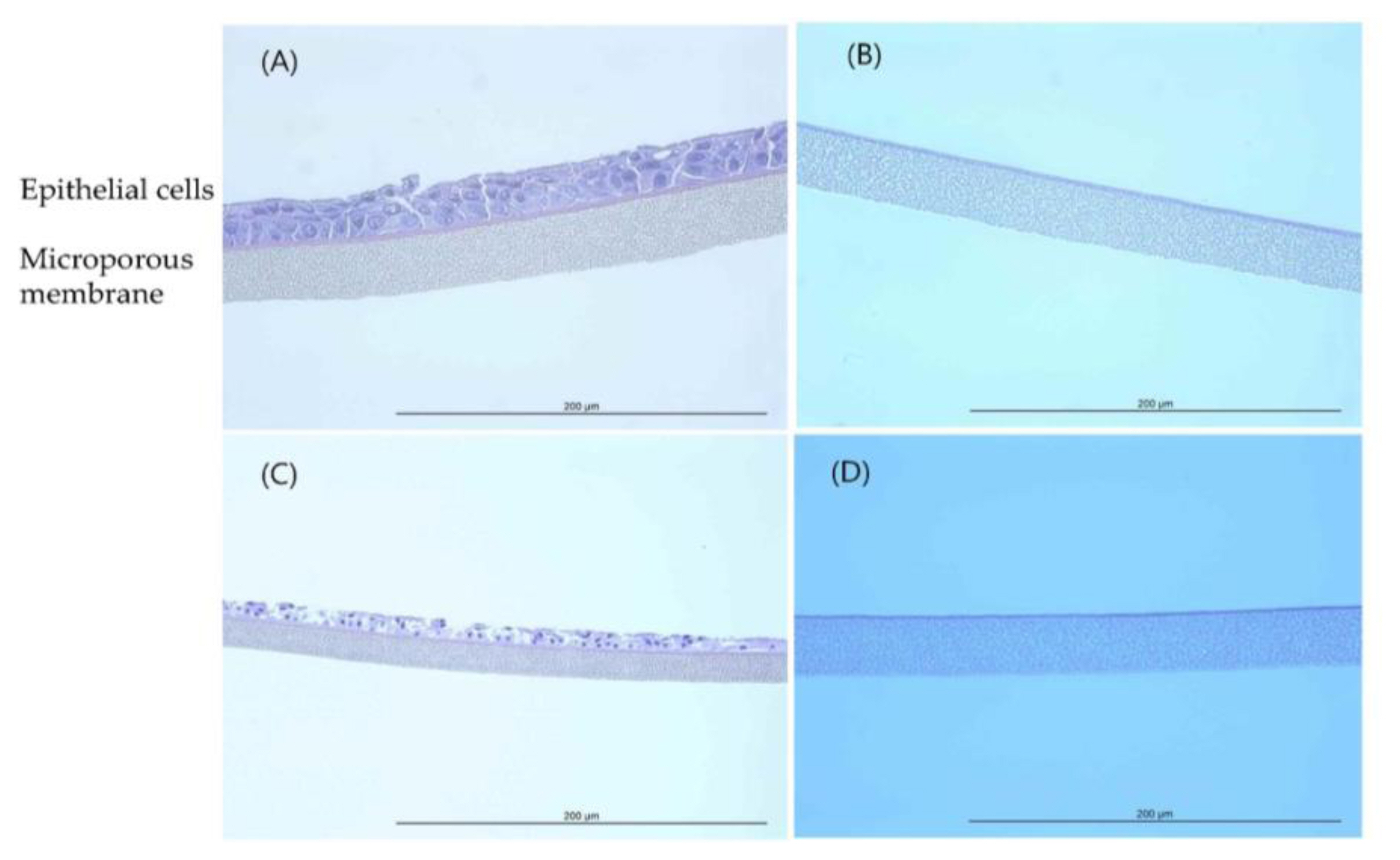
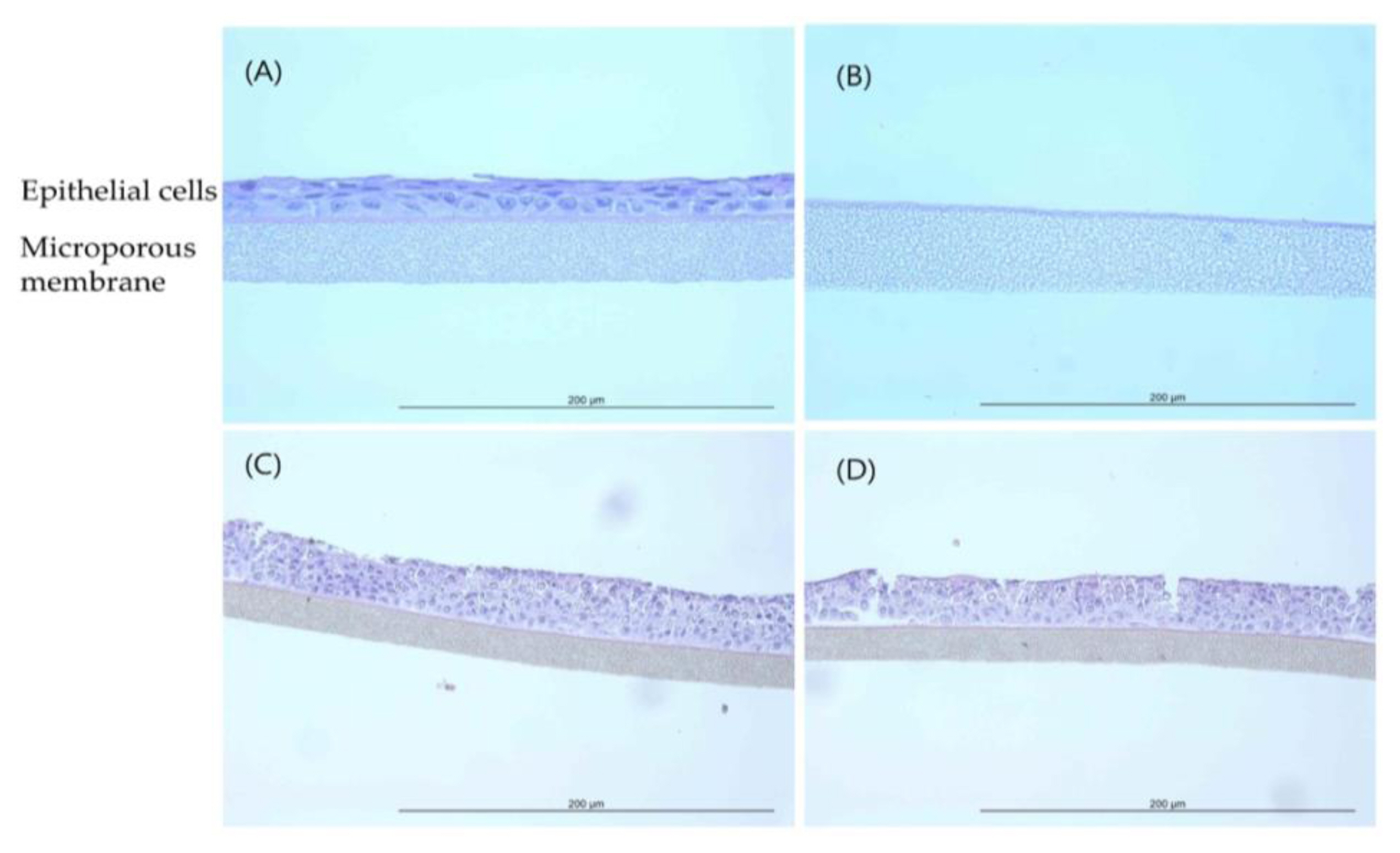
Histopathological observations showed that the epithelial cell layer effects for TiO2 NPs (GST and P-25) were not observed in tissue model and it was similar with negative control (D.W). However, the cell layer was almost entirely damaged and eliminated in positive control (formaldehyde solution). These changes in the histopathological examination were observed to be similar to results of MTT tissue viability (%). The histopathological findings were presented in (Figure 4 and Figure 5).
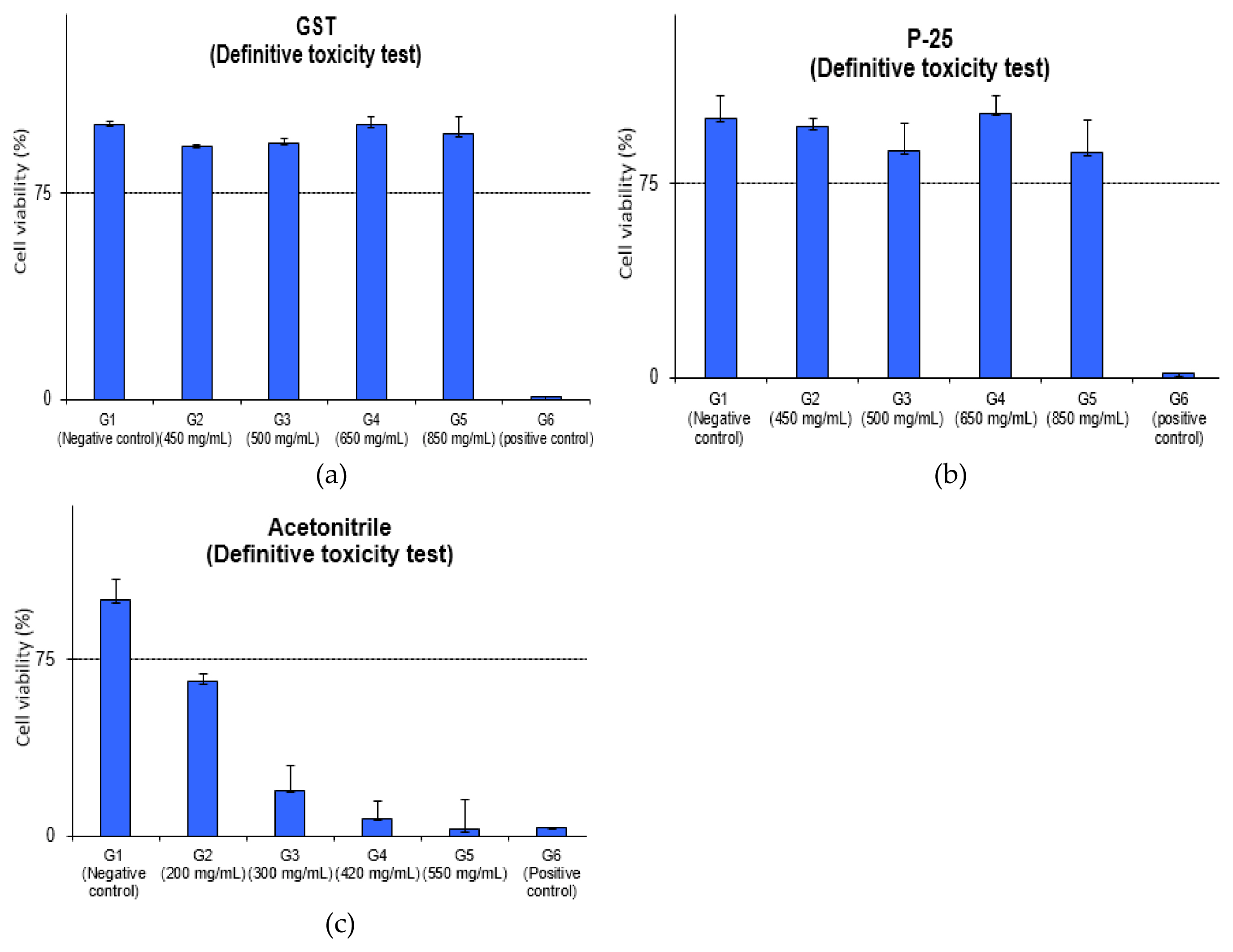
Definitive toxicity test of relative viability: (a) GST of relative viability; (b) P-25 of relative viability; (c) acetonitrile of relative viability.

EpiAirway™ tissue exposed to GST (H&E, HPF 400×): (A) Negative control (D.W); (B) Positive control (Formaldehyde solution); (C) 400 mg/mL concentration; (D) 850 mg/mL concentration.
In tissue observation for acetonitrile, the damages for cell layer were confirmed in 200 mg/mL and 550 mg/mL, respectively as (Figure 6) (C, D). These effects showed dose-dependent tendency and was similar to results of MTT tissue viability (%); destruction of cell layer in 200 mg/mL concentration, elimination of cell layer in 550 mg/mL (Figure 7).
EpiAirway™ tissue exposed to P-25 (H&E, HPF 400×): (A) Negative control (20% Acetone, acetone/olive-oil (1:4)); (B) Positive control (Formaldehyde solution); (C) 400 mg/mL concentration; (D) 850 mg/mL concentration.
Conclusions
When the TiO2 NPs (GST) was tested with an EpiAirway™ tissue model (human tracheal/bronchial tissue) as a screening method for in vitro acute inhalation toxicity, it was concluded that IC75 could not be confirmed up to 850 mg/mL in MTT viability and no histopathological effect was observed for tissue model. Also, EpiAirway™ model application appears to be a promising approach for in vitro determination of acute inhalation toxicity testing to facilitate moderately high-throughput screening. These results for GST were similar with that of P-25, commercial product under this condition. Therefore, on the basis of results of in vitro tissue model for GST manufactured as a photocatalyst through of sludge recycling, it was considered that GST could be classified as non-hazardous substance in inhalation route.
Acknowledgement
This work was supported by a grant (19SCIP-B145906-02) from the Korea Agency for Infrastructure Technology Advancement (KAIA) by Ministry of Land, Infrastructure and Transport of Korea government (MOLIT), Republic of Korea.
Notes
The authors declare that they have no conflict of interest.
CRediT author statement
SYJ: Methodology, Data curation, Writing-Original draft preparation; MKP: Supervision, Writing-Reviewing and Editing; JMI: Visualization; HSP: Visualization; HSS: Visualization; HJP: Resources; SSN: Project administration, Writing-Reviewing and Editing.